Adenylyl cyclase 3 regulates osteocyte mechanotransduction and primary cilium
- PMID: 34411897
- PMCID: PMC8406666
- DOI: 10.1016/j.bbrc.2021.08.033
Adenylyl cyclase 3 regulates osteocyte mechanotransduction and primary cilium
Abstract
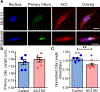
Osteocytes are accepted as the primary mechanosensing cell in bone, but how they translate mechanical signals into biochemical signals remains unclear. Adenylyl cyclases (AC) are enzymes that catalyze the production of second messenger cyclic adenosine monophosphate (cAMP). Osteocytes display a biphasic, cAMP response to fluid shear with an initial decrease in cAMP concentrations and then an increased concentration after sustained mechanical stimulation. To date, AC6, a calcium-inhibited AC, is the primary isoform studied in bone. Since osteocytes are calcium-responsive mechanosensors, we asked if a calcium-stimulated isoform contributes to mechanotransduction. Using a transcriptomic dataset of MLO-Y4 osteocyte-like cells from the NIH Gene Expression Omnibus, we identified AC3 as the only calcium-stimulated isoform expressed. We show that inhibiting AC3 in MLO-Y4 cells results in decreased cAMP-signaling with fluid shear and increased osteogenic response to fluid flow (measured as Ptgs2 expression) of longer durations, but not shorter. AC3 likely contributes to osteocyte mechanotransduction through a signaling axis involving the primary cilium and GSK3β. We demonstrate that AC3 localizes to the primary cilium, as well as throughout the cytosol and that fluid-flow regulation of primary cilia length is altered with an AC3 knockdown. Regulation of GSK3β is downstream of the primary cilium and cAMP signaling, and with western blots we found that GSK3β inhibition by phosphorylation is increased after fluid shear in AC3 knockdown groups. Our data show that AC3 contributes to osteocyte mechanotransduction and warrants further investigation to pave the way to identifying new therapeutic targets to treat bone disease like osteoporosis.
Keywords: Adenylyl cyclase 3; Gsk3beta; Mechanotransduction; Osteocyte; Primary cilium; cAMP.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
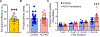
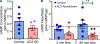
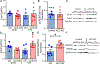
Similar articles
-
Osteocytes and Primary Cilia.Curr Osteoporos Rep. 2023 Dec;21(6):719-730. doi: 10.1007/s11914-023-00819-1. Epub 2023 Sep 8. Curr Osteoporos Rep. 2023. PMID: 37682373 Free PMC article. Review.
-
Primary cilium-dependent mechanosensing is mediated by adenylyl cyclase 6 and cyclic AMP in bone cells.FASEB J. 2010 Aug;24(8):2859-68. doi: 10.1096/fj.09-148007. Epub 2010 Apr 6. FASEB J. 2010. PMID: 20371630 Free PMC article.
-
Adenylyl cyclases and TRPV4 mediate Ca2+/cAMP dynamics to enhance fluid flow-induced osteogenesis in osteocytes.J Mol Biochem. 2018;7:48-59. J Mol Biochem. 2018. PMID: 31123666 Free PMC article.
-
PTH1R translocation to primary cilia in mechanically-stimulated ostecytes prevents osteoclast formation via regulation of CXCL5 and IL-6 secretion.J Cell Physiol. 2022 Oct;237(10):3927-3943. doi: 10.1002/jcp.30849. Epub 2022 Aug 7. J Cell Physiol. 2022. PMID: 35933642 Free PMC article.
-
Emerging role of primary cilia as mechanosensors in osteocytes.Bone. 2013 Jun;54(2):196-204. doi: 10.1016/j.bone.2012.11.016. Epub 2012 Nov 28. Bone. 2013. PMID: 23201223 Free PMC article. Review.
Cited by
-
The Nociceptor Primary Cilium Contributes to Mechanical Nociceptive Threshold and Inflammatory and Neuropathic Pain.J Neurosci. 2024 Nov 20;44(47):e1265242024. doi: 10.1523/JNEUROSCI.1265-24.2024. J Neurosci. 2024. PMID: 39349056 Free PMC article.
-
Cilia in the brain display region-dependent oscillations of length and orientation.PLoS Biol. 2025 Jul 11;23(7):e3003197. doi: 10.1371/journal.pbio.3003197. eCollection 2025 Jul. PLoS Biol. 2025. PMID: 40644366 Free PMC article.
-
Circadian cilia transcriptome in mouse brain across physiological and pathological states.Mol Brain. 2024 Sep 20;17(1):67. doi: 10.1186/s13041-024-01143-0. Mol Brain. 2024. PMID: 39304885 Free PMC article.
-
Osteocytes and Primary Cilia.Curr Osteoporos Rep. 2023 Dec;21(6):719-730. doi: 10.1007/s11914-023-00819-1. Epub 2023 Sep 8. Curr Osteoporos Rep. 2023. PMID: 37682373 Free PMC article. Review.
References
-
- Kittikulsuth W, Stuart D, Hoek ANV, Stockand JD, Bugaj V, Mironova E, et al.Lack of an effect of collecting duct-specific deletion of adenylyl cyclase 3 on renal Na+ and water excretion or arterial pressure. American Journal of Physiology - Renal Physiology. 2014March15;306(6):F597–607. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

