National population prevalence of antibodies to SARS-CoV-2 in Scotland during the first and second waves of the COVID-19 pandemic
- PMID: 34411992
- PMCID: PMC8289625
- DOI: 10.1016/j.puhe.2021.07.006
National population prevalence of antibodies to SARS-CoV-2 in Scotland during the first and second waves of the COVID-19 pandemic
Abstract
Objectives: Studies that measure the prevalence of antibodies to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) ('seroprevalence') are essential to understand population exposure to SARS-CoV-2 among symptomatic and asymptomatic individuals. We aimed to measure seroprevalence in the Scottish population over the course of the COVID-19 pandemic - from before the first recorded case in Scotland through to the second pandemic wave.
Study design: The study design of this study is serial cross sectional.
Methods: We tested 41,477 residual samples retrieved from primary and antenatal care settings across Scotland for SARS-CoV-2 antibodies over a 12-month period from December 2019-December 2020 (before rollout of COVID-19 vaccination). Five-weekly rolling seroprevalence estimates were adjusted for the sensitivity and specificity of the assays and weighted to reference populations. Temporal trends in seroprevalence estimates and weekly SARS-CoV-2 notifications were compared.
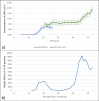
Results: Five-weekly rolling seroprevalence rates were 0% until the end of March, when they increased contemporaneously with the first pandemic wave. Seroprevalence rates remained stable through the summer (range: 3%-5%) during a period of social restrictions, after which they increased concurrently with the second wave, reaching 9.6% (95% confidence interval [CI]: 8.4%-10.8%) in the week beginning 28th December in 2020. Seroprevalence rates were lower in rural vs. urban areas (adjusted odds ratio [AOR]: 0.70, 95% CI: 0.61-0.79) and among individuals aged 20-39 years and 60 years and older (AOR: 0.74, 95% CI: 0.64-0.86; AOR: 0.80, 95% CI: 0.69-0.91, respectively) relative to those aged 0-19 years.
Conclusions: After two waves of the COVID-19 pandemic, less than one in ten individuals in the Scottish population had antibodies to SARS-CoV-2. Seroprevalence may underestimate the true population exposure as a result of waning antibodies among individuals who were infected early in the first wave.
Keywords: Antibodies; COVID-19; Cross sectional; SARS-CoV-2; Seroprevalence.
Copyright © 2021 The Royal Society for Public Health. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
P.M. owns shares of Astra Zeneca. The remaining authors have no competing interests to declare.
Figures
References
-
- Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020 Aug 15;396(10249):467–478. - PMC - PubMed
-
- Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020 Aug 12;586(7830):589–593. - PubMed
-
- Diggle P.J. Estimating prevalence using an imperfect test. Epidemiol Res Int. 2011;2011:1–5.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

