ATR Inhibitor M6620 (VX-970) Enhances the Effect of Radiation in Non-Small Cell Lung Cancer Brain Metastasis Patient-Derived Xenografts
- PMID: 34413128
- PMCID: PMC8571002
- DOI: 10.1158/1535-7163.MCT-21-0305
ATR Inhibitor M6620 (VX-970) Enhances the Effect of Radiation in Non-Small Cell Lung Cancer Brain Metastasis Patient-Derived Xenografts
Abstract
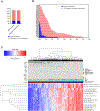
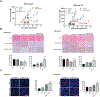
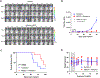
M6620, a selective ATP-competitive inhibitor of the ATM and RAD3-related (ATR) kinase, is currently under investigation with radiation in patients with non-small cell lung cancer (NSCLC) brain metastases. We evaluated the DNA damage response (DDR) pathway profile of NSCLC and assessed the radiosensitizing effects of M6620 in a preclinical NSCLC brain metastasis model. Mutation analysis and transcriptome profiling of DDR genes and pathways was performed on NSCLC patient samples. NSCLC cell lines were assessed with proliferation, clonogenic survival, apoptosis, cell cycle, and DNA damage signaling and repair assays. NSCLC brain metastasis patient-derived xenograft models were used to assess intracranial response and overall survival. In vivo IHC was performed to confirm in vitro results. A significant portion of NSCLC patient tumors demonstrated enrichment of DDR pathways. DDR pathways correlated with lung squamous cell histology; and mutations in ATR, ATM, BRCA1, BRCA2, CHEK1, and CHEK2 correlated with enrichment of DDR pathways in lung adenocarcinomas. M6620 reduced colony formation after radiotherapy and resulted in inhibition of DNA DSB repair, abrogation of the radiation-induced G2 cell checkpoint, and formation of dysfunctional micronuclei, leading to enhanced radiation-induced mitotic death. The combination of M6620 and radiation resulted in improved overall survival in mice compared with radiation alone. In vivo IHC revealed inhibition of pChk1 in the radiation plus M6620 group. M6620 enhances the effect of radiation in our preclinical NSCLC brain metastasis models, supporting the ongoing clinical trial (NCT02589522) evaluating M6620 in combination with whole brain irradiation in patients with NSCLC brain metastases.
©2021 American Association for Cancer Research.
Conflict of interest statement
No potential conflicts of interest were disclosed by the other authors.
Figures


References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: A Cancer Journal for Clinicians. 2020;70:7–30. - PubMed
-
- Sun A, Hu C, Wong SJ, Gore E, Videtic G, Dutta S, et al. Prophylactic Cranial Irradiation vs Observation in Patients With Locally Advanced Non-Small Cell Lung Cancer: A Long-term Update of the NRG Oncology/RTOG 0214 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2019/03/15 ed. 2019;5:847–55. - PMC - PubMed
-
- Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, et al. Stereotactic Radiosurgery Plus Whole-Brain Radiation Therapy vs Stereotactic Radiosurgery Alone for Treatment of Brain Metastases: A Randomized Controlled Trial. JAMA. American Medical Association; 2006;295:2483–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

