Autoantibodies neutralizing type I IFNs are present in ~ 4% of uninfected individuals over 70 years old and account for ~ 20% of COVID-19 deaths
- PMID: 34413139
- PMCID: PMC8521484
- DOI: 10.1126/sciimmunol.abl4340
Autoantibodies neutralizing type I IFNs are present in ~ 4% of uninfected individuals over 70 years old and account for ~ 20% of COVID-19 deaths
Abstract
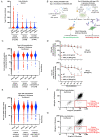
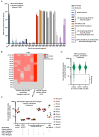
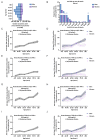
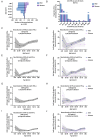
Circulating autoantibodies (auto-Abs) neutralizing high concentrations (10 ng/mL, in plasma diluted 1 to 10) of IFN-α and/or -ω are found in about 10% of patients with critical COVID-19 pneumonia, but not in subjects with asymptomatic infections. We detect auto-Abs neutralizing 100-fold lower, more physiological, concentrations of IFN-α and/or -ω (100 pg/mL, in 1/10 dilutions of plasma) in 13.6% of 3,595 patients with critical COVID-19, including 21% of 374 patients > 80 years, and 6.5% of 522 patients with severe COVID-19. These antibodies are also detected in 18% of the 1,124 deceased patients (aged 20 days-99 years; mean: 70 years). Moreover, another 1.3% of patients with critical COVID-19 and 0.9% of the deceased patients have auto-Abs neutralizing high concentrations of IFN-β. We also show, in a sample of 34,159 uninfected subjects from the general population, that auto-Abs neutralizing high concentrations of IFN-α and/or -ω are present in 0.18% of individuals between 18 and 69 years, 1.1% between 70 and 79 years, and 3.4% >80 years. Moreover, the proportion of subjects carrying auto-Abs neutralizing lower concentrations is greater in a subsample of 10,778 uninfected individuals: 1% of individuals <70 years, 2.3% between 70 and 80 years, and 6.3% >80 years. By contrast, auto-Abs neutralizing IFN-β do not become more frequent with age. Auto-Abs neutralizing type I IFNs predate SARS-CoV-2 infection and sharply increase in prevalence after the age of 70 years. They account for about 20% of both critical COVID-19 cases in the over-80s, and total fatal COVID-19 cases.
Copyright © 2021, American Association for the Advancement of Science.
Figures
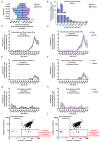
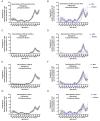
Comment in
-
Rogue antibodies involved in almost one-fifth of COVID deaths.Nature. 2021 Sep;597(7875):162. doi: 10.1038/d41586-021-02337-5. Nature. 2021. PMID: 34471247 No abstract available.
-
Transient Increase of Pre-existing Anti-IFN-α2 Antibodies Induced by SARS-CoV-2 Infection.J Clin Immunol. 2022 May;42(4):742-745. doi: 10.1007/s10875-022-01235-3. Epub 2022 Mar 17. J Clin Immunol. 2022. PMID: 35296990 Free PMC article. No abstract available.
-
High frequency of type I interferon auto-antibodies in a group of middle-aged HIV-infected patients: A cross-sectional exploratory study.Immun Inflamm Dis. 2023 Nov;11(11):e1056. doi: 10.1002/iid3.1056. Immun Inflamm Dis. 2023. PMID: 38018592 Free PMC article.
References
-
- Levin A. T., Hanage W. P., Owusu-Boaitey N., Cochran K. B., Walsh S. P., Meyerowitz-Katz G., Assessing the age specificity of infection fatality rates for COVID-19: Systematic review, meta-analysis, and public policy implications. Eur. J. Epidemiol. 35, 1123–1138 (2020). 10.1007/s10654-020-00698-1 - DOI - PMC - PubMed
-
- Williamson E. J., Walker A. J., Bhaskaran K., Bacon S., Bates C., Morton C. E., Curtis H. J., Mehrkar A., Evans D., Inglesby P., Cockburn J., McDonald H. I., MacKenna B., Tomlinson L., Douglas I. J., Rentsch C. T., Mathur R., Wong A. Y. S., Grieve R., Harrison D., Forbes H., Schultze A., Croker R., Parry J., Hester F., Harper S., Perera R., Evans S. J. W., Smeeth L., Goldacre B., Factors associated with COVID-19-related death using OpenSAFELY. Nature 584, 430–436 (2020). 10.1038/s41586-020-2521-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 75N91019D00024/CA/NCI NIH HHS/United States
- S10 OD018521/OD/NIH HHS/United States
- 2U19 AI111825/NH/NIH HHS/United States
- U19 AI111825/AI/NIAID NIH HHS/United States
- U24 HG008956/HG/NHGRI NIH HHS/United States
- P01 AI138398-S1/NH/NIH HHS/United States
- 1ZIA AI001265/ImNIH/Intramural NIH HHS/United States
- ZIA AI001265/ImNIH/Intramural NIH HHS/United States
- UM1 HG006504/HG/NHGRI NIH HHS/United States
- MR/S032304/1/MRC_/Medical Research Council/United Kingdom
- R01 AI091707/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- UL1 TR001866/TR/NCATS NIH HHS/United States
- R01 AI088364/AI/NIAID NIH HHS/United States
- R01 AI091707-10S1/NH/NIH HHS/United States
- R01 AI163029/AI/NIAID NIH HHS/United States
- ZIA AI001270/ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

