Redox-enabled direct stereoconvergent heteroarylation of simple alcohols
- PMID: 34413301
- PMCID: PMC8376995
- DOI: 10.1038/s41467-021-25268-1
Redox-enabled direct stereoconvergent heteroarylation of simple alcohols
Abstract
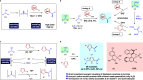
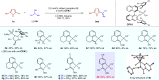
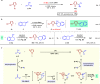
The direct transformation of racemic feedstock materials to valuable enantiopure compounds is of significant importance for sustainable chemical synthesis. Toward this goal, the radical mechanism has proven uniquely effective in stereoconvergent carbon-carbon bond forming reactions. Here we report a mechanistically distinct redox-enabled strategy for an efficient enantioconvergent coupling of pyrroles with simple racemic secondary alcohols. In such processes, chirality is removed from the substrate via dehydrogenation and reinstalled in the catalytic reduction of a key stabilized cationic intermediate. This strategy provides significant advantage of utilizing simple pyrroles to react with feedstock alcohols without the need for leaving group incorporation. This overall redox-neutral transformation is also highly economical with no additional reagent nor waste generation other than water. In our studies, oxime-derived iridacycle complexes are introduced, which cooperate with a chiral phosphoric acid to enable heteroarylation of alcohols, accessing a wide range of valuable substituted pyrroles in high yield and enantioselectivity.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Bhat V, Welin ER, Guo X, Stoltz BM. Advances in stereoconvergent catalysis from 2005 to 2015: transition-metal-mediated stereoablative reactions, dynamic kinetic resolutions, and dynamic kinetic asymmetric transformations. Chem. Rev. 2017;117:4528–4561. doi: 10.1021/acs.chemrev.6b00731. - DOI - PMC - PubMed
-
- Zhang X, Tan C-H. Stereospecific and stereoconvergent nucleophilic substitution reactions at tertiary carbon centers. Chem. 2021;7:1451–1486. doi: 10.1016/j.chempr.2020.11.022. - DOI
Publication types
LinkOut - more resources
Full Text Sources

