Structure activity relationships and the binding mode of quinolinone-pyrimidine hybrids as reversal agents of multidrug resistance mediated by P-gp
- PMID: 34413359
- PMCID: PMC8376931
- DOI: 10.1038/s41598-021-96226-6
Structure activity relationships and the binding mode of quinolinone-pyrimidine hybrids as reversal agents of multidrug resistance mediated by P-gp
Abstract
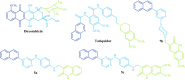
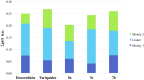
P-gp-associated multidrug resistance is a major impediment to the success of chemotherapy. With the aim of finding non-toxic and effective P-gp inhibitors, we investigated a panel of quinolin-2-one-pyrimidine hybrids. Among the active compounds, two of them significantly increased intracellular doxorubicin and rhodamine 123 accumulation by inhibiting the efflux mediated by P-gp and restored doxorubicin toxicity at nanomolar range. Structure-activity relationships showed that the number of methoxy groups, an optimal length of the molecule in its extended conformation, and at least one flexible methylene group bridging the quinolinone to the moiety bearing the pyrimidine favored the inhibitory potency of P-gp. The best compounds showed a similar binding pattern and interactions to those of doxorubicin and tariquidar, as revealed by MD and hybrid QM/MM simulations performed with the recent experimental structure of P-gp co-crystallized with paclitaxel. Analysis of the molecular interactions stabilizing the different molecular complexes determined by MD and QTAIM showed that binding to key residues from TMH 4-7 and 12 is required for inhibition.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
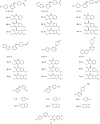
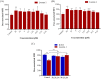
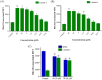
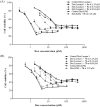




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

