A human forebrain organoid model of fragile X syndrome exhibits altered neurogenesis and highlights new treatment strategies
- PMID: 34413513
- PMCID: PMC8484073
- DOI: 10.1038/s41593-021-00913-6
A human forebrain organoid model of fragile X syndrome exhibits altered neurogenesis and highlights new treatment strategies
Abstract
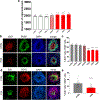
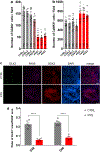
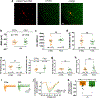
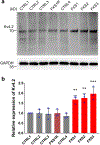
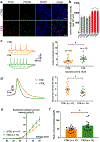
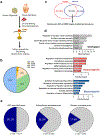
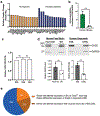
Fragile X syndrome (FXS) is caused by the loss of fragile X mental retardation protein (FMRP), an RNA-binding protein that can regulate the translation of specific mRNAs. In this study, we developed an FXS human forebrain organoid model and observed that the loss of FMRP led to dysregulated neurogenesis, neuronal maturation and neuronal excitability. Bulk and single-cell gene expression analyses of FXS forebrain organoids revealed that the loss of FMRP altered gene expression in a cell-type-specific manner. The developmental deficits in FXS forebrain organoids could be rescued by inhibiting the phosphoinositide 3-kinase pathway but not the metabotropic glutamate pathway disrupted in the FXS mouse model. We identified a large number of human-specific mRNAs bound by FMRP. One of these human-specific FMRP targets, CHD2, contributed to the altered gene expression in FXS organoids. Collectively, our study revealed molecular, cellular and electrophysiological abnormalities associated with the loss of FMRP during human brain development.
© 2021. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

