Targeting Tryptophan for Tagging Through Photo-induced Electron Transfer
- PMID: 34413573
- PMCID: PMC8372833
- DOI: 10.1055/a-1479-6366
Targeting Tryptophan for Tagging Through Photo-induced Electron Transfer
Abstract
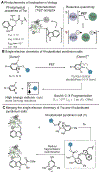
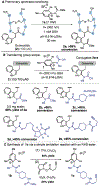
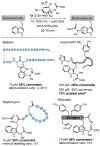
The chemical modification of tryptophan (Trp) has been the subject of interest for nearly 100 years, yet the development modification conditions that exploit Trp's inherent photolability have remained elusive. In this perspective, we discuss our recently reported method for Tryptophan (Trp) photobioconjugation that uses N -carbamoyl pyridinium salts to engage Trp in photo-induced electron transfer. We detail our inspiration and rationale as well as place our report in the context of select prior art in the field.
Keywords: Bioconjugation; Photo-induced Electron Transfer; Photochemistry; Proteins; Tryptophan.
Figures
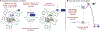


References
-
- Hermanson G Bioconjugate Techniques, 3rd ed.; Elsevier: London, 2013.
-
- Brotzel F; Mayer H Org. Biomol. Chem 2007, 5, 3814. - PubMed
-
- Turro NJ; Ramamurthy V; Scaiano JC Modern Molecular Photochemistry of Organic Molecules. Turro. University Science Books; Sausalito, 2010.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
