Exploring Markers of Exhausted CD8 T Cells to Predict Response to Immune Checkpoint Inhibitor Therapy for Hepatocellular Carcinoma
- PMID: 34414122
- PMCID: PMC8339511
- DOI: 10.1159/000515305
Exploring Markers of Exhausted CD8 T Cells to Predict Response to Immune Checkpoint Inhibitor Therapy for Hepatocellular Carcinoma
Abstract
Background: Reversal of CD8 T-cell exhaustion was considered a major antitumor mechanism of anti-programmed cell death-1 (PD-1)/ anti-programmed death ligand-1 (PD-L1)-based immune checkpoint inhibitor (ICI) therapy.
Objectives: The aim of this study was to identify markers of T-cell exhaustion that is best associated with ICI treatment efficacy for advanced hepatocellular carcinoma (HCC).
Methods: Immune cell composition of archival tumor samples was analyzed by transcriptomic analysis and multiplex immunofluorescence staining.
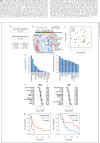
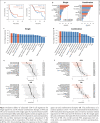
Results: HCC patients with objective response after anti-PD-1/anti-PD-L1-based ICI therapy (n = 42) had higher expression of genes related to T-cell exhaustion. A 9-gene signature (LAG3, CD244, CCL5, CXCL9, CXCL13, MSR1, CSF3R, CYBB, and KLRK1) was defined, whose expression was higher in patients with response to ICI therapy, correlated with density of CD8+LAG3+ cells in tumor microenvironment, and independently predicted better progression-free and overall survival. This 9-gene signature had similar predictive values for patients who received single-agent or combination ICI therapy and was not associated with prognosis in HCC patients who received surgery, suggesting that it may outperform other T-cell signatures for predicting efficacy of ICI therapy for HCC. For HCC patients who underwent surgery for both the primary liver and metastatic lung tumors (n = 31), lung metastatic HCC was associated with a higher exhausted CD8 T-cell signature, consistent with prior observation that patients with lung metastatic HCC may have higher probability of response to ICI therapy.
Conclusions: CD8 T-cell exhaustion in tumor microenvironment may predict better efficacy of ICI therapy for HCC.
Keywords: Anti-PD-1; Anti-PD-L1; Immune checkpoint inhibitor; T-cell exhaustion; Tumor microenvironment.
Copyright © 2021 by S. Karger AG, Basel.
Conflict of interest statement
Dr. Ann-Lii Cheng is a consultant for and a member of the speaker's bureau of Bayer-Schering Pharma. Dr. Ann-Lii Cheng is a consultant of Novartis, Merck Serono, Eisai, Merck Sharp & Dohme (MSD) Corp., ONXEO, Bayer HealthCare Pharmaceuticals Inc., BMS Company, and Ono Pharmaceutical Co., Ltd. Dr. Ann-Lii Cheng is an associate editor of Liver Cancer. Dr. Chiun Hsu received research grants from BMS/ONO, Roche, and Ipsen and received honorarium from the following pharmaceutical companies: AstraZeneca, Bayer, BMS/ONO, Eisai, Eli Lilly, Ipsen, Merck Serono, MSD, Novartis, Roche, TTY Biopharm.
Figures




References
-
- Callahan MK, Postow MA, Wolchok JD. Targeting T cell co-receptors for cancer therapy. Immunity. 2016;44((5)):1069–78. - PubMed
-
- Hoos A. Development of immuno-oncology drugs: from CTLA4 to PD1 to the next generations. Nat Rev Drug Discov. 2016;15((4)):235–47. - PubMed
-
- Zhu AX, Richard SF, Julien E, Stephane C, Sadahisa O, Daniel P, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19((7)):940–52. - PubMed
-
- Yau T, Park JW, Finn RS, Cheng A-L, Mathurin P, Edeline J, et al. CheckMate 459: A randomized, multi-center phase 3 study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC) Ann Oncol. 2019;30((Suppl 5)):v851–v934.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

