Chronic Hepatitis C Pathogenesis: Immune Response in the Liver Microenvironment and Peripheral Compartment
- PMID: 34414132
- PMCID: PMC8369367
- DOI: 10.3389/fcimb.2021.712105
Chronic Hepatitis C Pathogenesis: Immune Response in the Liver Microenvironment and Peripheral Compartment
Abstract
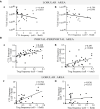
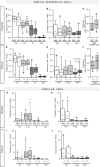
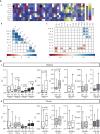
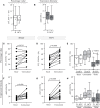
Chronic hepatitis C (CHC) pathogenic mechanisms as well as the participation of the immune response in the generation of liver damage are still a topic of interest. Here, we evaluated immune cell populations and cytokines in the liver and peripheral blood (PB) to elucidate their role in CHC pathogenesis. B, CTL, Th, Treg, Th1, Th17, and NK cell localization and frequency were evaluated on liver biopsies by immunohistochemistry, while frequency, differentiation, and functional status on PB were evaluated by flow cytometry. TNF-α, IL-23, IFN-γ, IL-1β, IL-6, IL-8, IL-17A, IL-21, IL-10, and TGF-β expression levels were quantified in fresh liver biopsy by RT-qPCR and in plasma by CBA/ELISA. Liver CTL and Th1 at the lobular area inversely correlated with viral load (r = -0.469, p =0.003 and r = -0.384, p = 0.040). Treg correlated with CTL and Th1 at the lobular area (r = 0.784, p < 0.0001; r = 0.436, p = 0.013). Th17 correlated with hepatic IL-8 (r = 0.52, p < 0.05), and both were higher in advanced fibrosis cases (Th17 p = 0.0312, IL-8 p = 0.009). Hepatic cytokines were higher in severe hepatitis cases (IL-1β p = 0.026, IL-23 p = 0.031, IL-8 p = 0.002, TGF-β, p= 0.037). Peripheral NK (p = 0.008) and NK dim (p = 0.018) were diminished, while NK bright (p = 0.025) was elevated in patients vs. donors. Naïve Th (p = 0.011) and CTL (p = 0.0007) were decreased, while activated Th (p = 0.0007) and CTL (p = 0.0003) were increased. IFN-γ production and degranulation activity in NK and CTL were normal. Peripheral cytokines showed an altered profile vs. donors, particularly elevated IL-6 (p = 0.008) and TGF-β (p = 0.041). Total hepatic CTLs favored damage. Treg could not prevent fibrogenesis triggered by Th17 and IL-8. Peripheral T-lymphocyte differentiation stage shift, elevated cytokine levels and NK-cell count decrease would contribute to global disease.
Keywords: HCV: Hepatitis C virus; Th17; Treg: regulatory T lymphocytes; chronic liver disease; immunopathogenesis; inflammatory liver infiltrate; peripheral blood.
Copyright © 2021 Rios, Casciato, Caldirola, Gaillard, Giadans, Ameigeiras, De Matteo, Preciado and Valva.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

