Mixed Acid Fermentation of Carbohydrate-Rich Dairy Manure Hydrolysate
- PMID: 34414173
- PMCID: PMC8370043
- DOI: 10.3389/fbioe.2021.724304
Mixed Acid Fermentation of Carbohydrate-Rich Dairy Manure Hydrolysate
Abstract
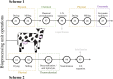
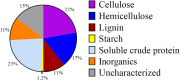
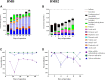
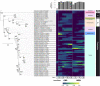
Dairy manure (DM) is an abundant agricultural residue that is largely composed of lignocellulosic biomass. The aim of this study was to investigate if carbon derived from DM fibers can be recovered as medium-chain fatty acids (MCFAs), which are mixed culture fermentation products of economic interest. DM fibers were subjected to combinations of physical, enzymatic, chemical, and thermochemical pretreatments to evaluate the possibility of producing carbohydrate-rich hydrolysates suitable for microbial fermentation by mixed cultures. Among the pretreatments tested, decrystalization dilute acid pretreatment (DCDA) produced the highest concentrations of glucose and xylose, and was selected for further experiments. Bioreactors fed DCDA hydrolysate were operated. Acetic acid and butyric acid comprised the majority of end products during operation of the bioreactors. MCFAs were transiently produced at a maximum concentration of 0.17 mg CODMCFAs/mg CODTotal. Analyses of the microbial communities in the bioreactors suggest that lactic acid bacteria, Megasphaera, and Caproiciproducens were involved in MCFA and C4 production during DCDA hydrolysate metabolism.
Keywords: biomass pretreatment; dairy manure; lignocellulosic biomass; medium-chain fatty acids; mixed culture fermentation.
Copyright © 2021 Ingle, Fortney, Walters, Donohue and Noguera.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Agati V., Guyot J. P., Morlon‐Guyot J., Talamond P., Hounhouigan D. J. (1998). Isolation and Characterization of New Amylolytic Strains of Lactobacillus Fermentum from Fermented maize Doughs (Mawè and Ogi) from Benin. J. Appl. Microbiol. 85, 512–520. 10.1046/j.1365-2672.1998.853527.x - DOI
-
- Aguirre-Villegas H. A., Larson R. A. (2017). Evaluating Greenhouse Gas Emissions from Dairy Manure Management Practices Using Survey Data and Lifecycle Tools. J. Clean. Prod. 143, 169–179. 10.1016/j.jclepro.2016.12.133 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

