Nintedanib-Induced Renal Thrombotic Microangiopathy
- PMID: 34414215
- PMCID: PMC8339448
- DOI: 10.1159/000517692
Nintedanib-Induced Renal Thrombotic Microangiopathy
Abstract
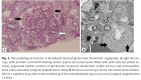
Nintedanib is a unique tyrosine kinase inhibitor used to suppress fibrosis in patients with idiopathic pulmonary fibrosis (IPF). Nintedanib has been shown to suppress multiple processes of fibrosis, thereby reducing the rate of lung function decline in patients with IPF. Since vascular endothelial growth factor is one of this agent's targets, nephrotoxicity, including renal thrombotic microangiopathy (TMA), is a possible major adverse effect. However, only 2 previous cases of nintedanib-induced renal TMA have been published. Our patient was an 83-year-old man with IPF. As adverse effects including liver enzyme level elevation, diarrhoea, anorexia, and nephrotoxicity developed, the nintedanib dosage was reduced after 9 months. The digestive symptoms resolved promptly, but the proteinuria and reduced kidney function remained. Although the kidney injury had improved to some extent, we performed a percutaneous renal biopsy. The biopsy revealed typical TMA findings such as microaneurysms filled with pale material, segmental double contours of glomerular basement membranes, and intracapillary foam cells. After discontinuation of nintedanib, the patient's nephrotoxicity improved. Nintedanib-induced renal TMA is reversible and is possibly dose-dependent. Here, we report the clinical course of our case and review the characteristics of nintedanib-induced renal TMA.
Keywords: Drug-induced kidney injury; Idiopathic pulmonary fibrosis; Nephrotic syndrome; Nintedanib; Proteinuria; Thrombotic microangiopathy; Tyrosine kinase inhibitor.
Copyright © 2021 by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

References
-
- Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis. 2007 Feb;49((2)):186–93. - PubMed
-
- Ranpura V, Pulipati B, Chu D, Zhu X, Wu S. Increased risk of high-grade hypertension with bevacizumab in cancer patients: a meta-analysis. Am J Hypertens. 2010 May;23((5)):460–8. - PubMed
-
- Cheungpasitporn W, Chebib FT, Cornell LD, Brodin ML, Nasr SH, Schinstock CA, et al. Intravitreal antivascular endothelial growth factor therapy may induce proteinuria and antibody mediated injury in renal allografts. Transplantation. 2015 Nov;99((11)):2382–6. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

