Bone Marrow Neutrophils of Multiple Myeloma Patients Exhibit Myeloid-Derived Suppressor Cell Activity
- PMID: 34414242
- PMCID: PMC8369183
- DOI: 10.1155/2021/6344344
Bone Marrow Neutrophils of Multiple Myeloma Patients Exhibit Myeloid-Derived Suppressor Cell Activity
Abstract
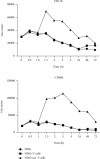
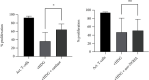
Activated normal density granulocytes (NDGs) can suppress T-cell responses in a similar way as myeloid-derived suppressor cells (MDSCs). In this study, we tested the hypothesis that NDGs from blood and bone marrow of multiple myeloma (MM) patients have the ability to suppress T-cells, as MDSC. MM is an incurable plasma cell malignancy of the bone marrow. Like most malignancies, myeloma cells alter its microenvironment to promote tumor growth, including inhibition of the immune system. We found that MM NDG from the bone marrow suppressed proliferation of T-cells, in contrast to healthy donors. The inhibitory effect could not be explained by changed levels of mature or immature NDG in the bone marrow. Moreover, NDG isolated from the blood of both myeloma patients and healthy individuals could inhibit T-cell proliferation and IFN-γ production. On the contrary to previous studies, blood NDGs did not have to be preactivated to mediate suppressive effects. Instead, they became activated during coculture, indicating that contact with activated T-cells is important for their ability to regulate T-cells. The inhibitory effect was dependent on the production of reactive oxygen species and could be reverted by the addition of its inhibitor, catalase. Our findings suggest that blood NDGs from MM patients are suppressive, but no more than NDGs from healthy donors. However, only bone marrow NDG from MM patients exhibited MDSC function. This MDSC-like suppression mediated by bone marrow NDG could be important for the growth of malignant plasma cells in MM patients.
Copyright © 2021 Julia Petersson et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

