Isolation and analysis methods of extracellular vesicles (EVs)
- PMID: 34414401
- PMCID: PMC8372011
- DOI: 10.20517/evcna.2021.07
Isolation and analysis methods of extracellular vesicles (EVs)
Erratum in
-
Erratum: Isolation and analysis methods of extracellular vesicles (EVs).Extracell Vesicles Circ Nucl Acids. 2021 Sep 15;2(3):222-223. doi: 10.20517/evcna.2021.15. eCollection 2021. Extracell Vesicles Circ Nucl Acids. 2021. PMID: 39697590 Free PMC article.
Abstract
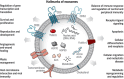
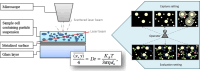
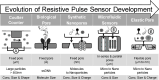
Extracellular vesicles (EVs) have been recognized as an evolving biomarker within the liquid biopsy family. While carrying both host cell proteins and different types of RNAs, EVs are also present in sufficient quantities in biological samples to be tested using many molecular analysis platforms to interrogate their content. However, because EVs in biological samples are comprised of both disease and non-disease related EVs, enrichment is often required to remove potential interferences from the downstream molecular assay. Most benchtop isolation/enrichment methods require > milliliter levels of sample and can cause varying degrees of damage to the EVs. In addition, some of the common EV benchtop isolation methods do not sort the diseased from the non-diseased related EVs. Simultaneously, the detection of the overall concentration and size distribution of the EVs is highly dependent on techniques such as electron microscopy and Nanoparticle Tracking Analysis, which can include unexpected variations and biases as well as complexity in the analysis. This review discusses the importance of EVs as a biomarker secured from a liquid biopsy and covers some of the traditional and non-traditional, including microfluidics and resistive pulse sensing, technologies for EV isolation and detection, respectively.
Keywords: Extracellular vesicles; microfluidics; molecular cargo; nanoparticle tracking analysis; resistive pulse sensing.
Conflict of interest statement
Conflicts of interest All authors declared that there are no conflicts of interest.
Figures







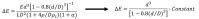
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
