Phase 1 study in healthy participants of the safety, pharmacokinetics, and pharmacodynamics of enpatoran (M5049), a dual antagonist of toll-like receptors 7 and 8
- PMID: 34414672
- PMCID: PMC8377444
- DOI: 10.1002/prp2.842
Phase 1 study in healthy participants of the safety, pharmacokinetics, and pharmacodynamics of enpatoran (M5049), a dual antagonist of toll-like receptors 7 and 8
Abstract
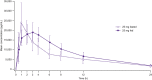
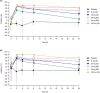
This study evaluated the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of single and multiple oral doses of enpatoran (formerly named M5049), a new toll-like receptor (TLR) 7 and 8 dual antagonist, and the effect of food on a single dose in healthy participants. In this single phase 1, randomized (3:1), double-blind, placebo-controlled study, 96 participants received single and multiple ascending oral doses of enpatoran. Participants in single-dose cohorts received one dose of enpatoran (1, 3, 9, 25, 50, 100, or 200 mg) or placebo using a sentinel dosing strategy. Multiple-dose cohorts received enpatoran (9, 25, or 200 mg once daily, or 25 or 50 mg twice daily) or placebo for 14 days. Safety, tolerability, PK, and PD (ex vivo-stimulated cytokine secretion) were assessed in both parts. The effect of food was assessed in an open-label, one-way crossover study in the 25 mg single-dose cohort. Single- and multiple-oral doses of enpatoran up to 200 mg were well tolerated and no significant dose-limiting adverse events or safety signals were observed under fasting or fed conditions. PK parameters were linear and dose-proportional across the dose range evaluated, with a slightly delayed absorption and lower peak concentration observed at 25 mg with food. Exposure-dependent inhibition of ex vivo-stimulated interleukin-6 secretion was observed, with maximum inhibition at 200 mg. Enpatoran was well tolerated at doses up to 200 mg. Further investigation of enpatoran is warranted as a potential treatment for diseases driven by TLR7/8 overactivation, such as systemic lupus erythematosus and COVID-19 pneumonia.
Keywords: autoimmune diseases; pharmacodynamics; pharmacokinetics; safety; toll-like receptors.
© 2021 The Authors. Pharmacology Research & Perspectives published by British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics and John Wiley & Sons Ltd.
Conflict of interest statement
AP, LKS, ABy, Aba, and CV are employees of the healthcare business of Merck KGaA, Darmstadt, Germany. JS and KG are employees of EMD Serono, Billerica, MA, USA. At the time of the study, VO was an employee of EMD Serono, Billerica, MA, USA. NM is an employee of Cytel, Paris, France. CR and DS are employees of Nuvisan GmbH, Neu‐Ulm, Germany. EH is a consultant for Nuventra, Inc.
Figures



References
-
- Zhang Z, Ohto U, Shibata T, et al. Structural analyses of toll‐like receptor 7 reveal detailed RNA sequence specificity and recognition mechanism of agonistic ligands. Cell Rep. 2018;25:3371‐3381.e5. - PubMed
-
- Bender AT, Tzvetkov E, Pereira A, et al. TLR7 and TLR8 differentially activate the IRF and NF‐kappaB pathways in specific cell types to promote inflammation. Immunohorizons. 2020;4:93‐107. - PubMed
-
- Gorden KB, Gorski KS, Gibson SJ, et al. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol. 2005;174:1259. - PubMed
-
- Larangé A, Antonios D, Pallardy M, Kerdine‐Römer S. TLR7 and TLR8 agonists trigger different signaling pathways for human dendritic cell maturation. J Leukoc Biol. 2009;85:673‐683. - PubMed
-
- de Marcken M, Dhaliwal K, Danielsen AC, Gautron AS, Dominguez‐Villar M. TLR7 and TLR8 activate distinct pathways in monocytes during RNA virus infection. Sci Signal. 2019;12:eaaw1347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

