Budesonide as induction therapy for incomplete microscopic colitis: A randomised, placebo-controlled multicentre trial
- PMID: 34414678
- PMCID: PMC8435258
- DOI: 10.1002/ueg2.12131
Budesonide as induction therapy for incomplete microscopic colitis: A randomised, placebo-controlled multicentre trial
Abstract
Background and aims: Incomplete microscopic colitis (MCi) is a subtype of microscopic colitis (MC). Budesonide is recommended as a first-line treatment for MC. However, randomised trials on efficacy of treatment in MCi are missing. We therefore performed a randomised, placebo-controlled trial to evaluate budesonide as induction therapy for MCi.
Methods: Patients with active MCi were randomly assigned to either budesonide 9 mg once daily or placebo for 8 weeks in a double-blind, double-dummy design. The primary endpoint was clinical remission, defined as a mean of <3 stools/day and a mean of <1 watery stool/day in the 7 days before week 8.
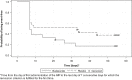
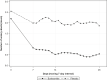
Results: Due to insufficient patient recruitment, the trial was discontinued prematurely. The intention-to-treat analysis included 44 patients (21 budesonide and 23 placebo). The primary endpoint of clinical remission at week 8 was obtained by 71.4% on budesonide and 43.5% on placebo (p = 0.0582). All clinical secondary endpoints were in favour of budesonide. Budesonide decreased the number of soft or watery stools (16.3 vs. 7.7, p = 0.0186) and improved health-related quality of life for all four dimensions of the short health scale. Adverse events with a suspected relation to study drug were reported in one patient in the budesonide group and two patients in the placebo group. Neither serious nor severe adverse events occurred during the double-blind phase.
Conclusions: Budesonide decreased the frequency of soft or watery stools and improved the patients' quality of life significantly in MCi, but the primary endpoint was not met due to the low sample size (type 2 error). Budesonide was safe and well tolerated during the 8-weeks treatment course.
Keywords: MCi; QoL; budesonide; drug; incomplete microscopic colitis; induction therapy; microscopic colitis; quality of life; randomised clinical trial; watery diarrhoea.
© 2021 The Authors. United European Gastroenterology Journal published by Wiley Periodicals LLC. on behalf of United European Gastroenterology.
Conflict of interest statement
Daniela Aust, Gerd Bouma, Juozas Kupčinskas, Ahmed Madisch, Stephan Miehlke and Andreas Münch have received consultancy honoraria or speaker's fees from Dr Falk Pharma GmbH, Freiburg, Germany. Ralf Mohrbacher and Roland Greinwald are employees of Dr Falk Pharma GmbH, Freiburg, Germany. All other authors have nothing to disclose.
Figures


References
-
- Read NW, Krejs GJ, Read MG, Santa Ana CA, Morawski SG, Fordtran JS. Chronic diarrhea of unknown origin. Gastroenterology. 1980;78:264–71. - PubMed
-
- Jaskiewicz K, Rzepko R, Adrych K, Smoczyński M. Microscopic colitis in routine colonoscopies. Dig Dis Sci. 2006;51:241–4. - PubMed
-
- Nyhlin N, Bohr J, Eriksson S, Tysk C. Microscopic colitis: a common and an easily overlooked cause of chronic diarrhoea. Eur J Intern Med. 2008;19:181–6. - PubMed
-
- Williams JJ, Kaplan GG, Makhija S, Urbanski SJ, Dupre M, Panaccione R, et al. Microscopic colitis‐defining incidence rates and risk factors: a population‐based study. Clin Gastroenterol Hepatol. 2008;6:35–40. - PubMed

