Mammalian lipids: structure, synthesis and function
- PMID: 34415021
- PMCID: PMC8578989
- DOI: 10.1042/EBC20200067
Mammalian lipids: structure, synthesis and function
Abstract
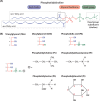
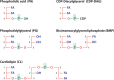
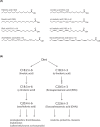
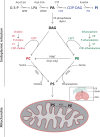
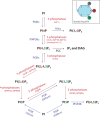
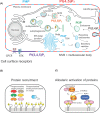
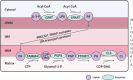
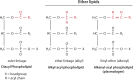
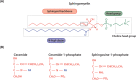
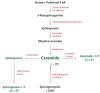
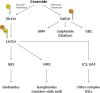
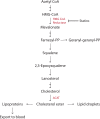
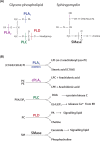
Lipids are essential constituents of cellular membranes. Once regarded merely as structural components, lipids have taken centre stage with the discovery of their roles in cell signalling and in the generation of bioactive metabolites. Lipids regulate many physiological functions of cells and alterations in membrane lipid metabolism are associated with major diseases including cancer, Type II diabetes, cardiovascular disease and immune disorders. Understanding lipid diversity, their synthesis and metabolism to generate signalling molecules will provide insight into the fundamental function of the cell. This review summarises the biosynthesis of the lipids of the mammalian cell; phospholipids, sphingolipids and cholesterol and how lipid diversity is achieved. The fatty acids (FAs) are the main building blocks of lipids and contribute to the diversity. Lipid synthesis is intimately connected to their transport within cells; the contribution by proteins that transport lipids, lipid transport proteins will be described. Cellular lipids are metabolised by phospholipases, lipid kinases and phosphatases to make new bioactive metabolites. These transient bioactive metabolites allow cells to respond to the external environment to maintain cellular health. The function of individual metabolites is also highlighted. Bioactive metabolites can be second messengers, or released to the external medium to regulate other cells. Alternatively, bioactive lipids also provide a platform for reversible recruitment of proteins to membranes using their lipid-binding domains. The wide range of physiological processes in which a specific involvement of lipids has been identified explains the need for lipid diversity present in mammalian cells.
Keywords: Cholesterol; Signalling; phosphatidylinositol; phospholipases; sphingolipids.
© 2021 The Author(s).
Conflict of interest statement
The author declares that there are no competing interests associated with the manuscript.
Figures



References
-
- Arnal-Levron M., Chen Y., Greimel P., Calevro F., Gaget K., Riols F.et al. . (2019) Bis(monoacylglycero)phosphate regulates oxysterol binding protein-related protein 11 dependent sterol trafficking. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 1864, 1247–1257 10.1016/j.bbalip.2019.05.011 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

