MCU overexpression evokes disparate dose-dependent effects on mito-ROS and spontaneous Ca2+ release in hypertrophic rat cardiomyocytes
- PMID: 34415186
- PMCID: PMC8794228
- DOI: 10.1152/ajpheart.00126.2021
MCU overexpression evokes disparate dose-dependent effects on mito-ROS and spontaneous Ca2+ release in hypertrophic rat cardiomyocytes
Abstract
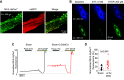
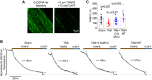
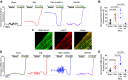
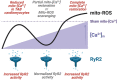
Cardiac dysfunction in heart failure (HF) and diabetic cardiomyopathy (DCM) is associated with aberrant intracellular Ca2+ handling and impaired mitochondrial function accompanied with reduced mitochondrial calcium concentration (mito-[Ca2+]). Pharmacological or genetic facilitation of mito-Ca2+ uptake was shown to restore Ca2+ transient amplitude in DCM and HF, improving contractility. However, recent reports suggest that pharmacological enhancement of mito-Ca2+ uptake can exacerbate ryanodine receptor-mediated spontaneous sarcoplasmic reticulum (SR) Ca2+ release in ventricular myocytes (VMs) from diseased animals, increasing propensity to stress-induced ventricular tachyarrhythmia. To test whether chronic recovery of mito-[Ca2+] restores systolic Ca2+ release without adverse effects in diastole, we overexpressed mitochondrial Ca2+ uniporter (MCU) in VMs from male rat hearts with hypertrophy induced by thoracic aortic banding (TAB). Measurement of mito-[Ca2+] using genetic probe mtRCamp1h revealed that mito-[Ca2+] in TAB VMs paced at 2 Hz under β-adrenergic stimulation is lower compared with shams. Adenoviral 2.5-fold MCU overexpression in TAB VMs fully restored mito-[Ca2+]. However, it failed to improve cytosolic Ca2+ handling and reduce proarrhythmic spontaneous Ca2+ waves. Furthermore, mitochondrial-targeted genetic probes MLS-HyPer7 and OMM-HyPer revealed a significant increase in emission of reactive oxygen species (ROS) in TAB VMs with 2.5-fold MCU overexpression. Conversely, 1.5-fold MCU overexpression in TABs, that led to partial restoration of mito-[Ca2+], reduced mitochondria-derived reactive oxygen species (mito-ROS) and spontaneous Ca2+ waves. Our findings emphasize the key role of elevated mito-ROS in disease-related proarrhythmic Ca2+ mishandling. These data establish nonlinear mito-[Ca2+]/mito-ROS relationship, whereby partial restoration of mito-[Ca2+] in diseased VMs is protective, whereas further enhancement of MCU-mediated Ca2+ uptake exacerbates damaging mito-ROS emission.NEW & NOTEWORTHY Defective intracellular Ca2+ homeostasis and aberrant mitochondrial function are common features in cardiac disease. Here, we directly compared potential benefits of mito-ROS scavenging and restoration of mito-Ca2+ uptake by overexpressing MCU in ventricular myocytes from hypertrophic rat hearts. Experiments using novel mito-ROS and Ca2+ biosensors demonstrated that mito-ROS scavenging rescued both cytosolic and mito-Ca2+ homeostasis, whereas moderate and high MCU overexpression demonstrated disparate effects on mito-ROS emission, with only a moderate increase in MCU being beneficial.
Keywords: calcium-dependent ventricular arrhythmia; mitochondrial calcium uniporter; mitochondrial calcium uptake; mitochondrial reactive oxygen species; ryanodine receptor.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures








References
-
- Hamilton S, Terentyeva R, Kim TY, Bronk P, Clements RT, O-Uchi J, Csordás G, Choi BR, Terentyev D. Pharmacological modulation of mitochondrial Ca2+ content regulates sarcoplasmic reticulum Ca2+ release via oxidation of the ryanodine receptor by mitochondria-derived reactive oxygen species. Front Physiol 9: 1831, 2018. doi:10.3389/fphys.2018.01831. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

