Effects of a disposable home electro-stimulation device (Pelviva) for the treatment of female urinary incontinence: a randomised controlled trial
- PMID: 34415401
- PMCID: PMC8377701
- DOI: 10.1007/s00404-021-06179-4
Effects of a disposable home electro-stimulation device (Pelviva) for the treatment of female urinary incontinence: a randomised controlled trial
Abstract
Aims: To compare current General Medical Practitioner treatment as usual (TAU) for the treatment of female urinary incontinence with a novel disposable home electro-stimulation device (Pelviva).
Methods: Open label, Primary Care post-market evaluation. 86 women with urinary incontinence were randomly assigned to one of two 12-week treatments: TAU or Pelviva for 30 min every other day plus TAU. Outcome measures included ICIQ-UI (primary), PISQ-IR, PGI-S / PGI-I and FSFI (secondary) at recruitment and immediately after intervention, 1-h pad test at recruitment and usage diaries throughout.
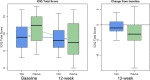
Results: Pelviva plus TAU produced significantly better outcome than TAU alone: 3 versus 1 point for ICIQ-UI (Difference - 1.8 95% CI: - 3.5 to - 0.1, P = 0.033). Significant differences were also observed for PGI-I at both 6 weeks (P = 0.001) and 12 weeks (P < 0.001). In the Pelviva group, 17% of women described themselves as feeling very much better and 54% a little or much better compared to 0% and 15% in the TAU. Overall PISQ-IR score reached statistical significance (P = 0.032) seemingly related to impact (P = 0.027). No other outcome measures reached statistical significance. Premature termination due to COVID-19 meant only 86 women were recruited from a sample size of 264. TAU did not reflect NICE guidelines.
Conclusions: This study suggests Pelviva is more successful than TAU in treating urinary incontinence in Primary Care. The study had reduced power due to early termination due to COVID-19 and suggests TAU does not follow NICE guidelines.
Keywords: Electrostimulation device; Female urinary incontinence; Pelvic floor muscle; Randomised Controlled Trial; Rehabilitation.
© 2021. Crown.
Conflict of interest statement
All authors received grant funding from Femeda for the submitted work and declare Jackie Oldham holds the patent for the electrical stimulation regimen incorporated in the Pelviva device. Julia Herbert was the Clinical Director to Femeda and received a consultancy fee from the Company.
Figures

Comment on
-
Evaluation of a new disposable "tampon like" electrostimulation technology (Pelviva®) for the treatment of urinary incontinence in women: a 12-week single blind randomized controlled trial.Neurourol Urodyn. 2013 Jun;32(5):460-6. doi: 10.1002/nau.22326. Epub 2012 Sep 28. Neurourol Urodyn. 2013. PMID: 23023996 Clinical Trial.
References
-
- Bo K, Frawley HC, Haylen BT, et al. An international urogynecological association (IUGA)/international continence society (ICS) joint report on the terminology for the conservative nonpharmacological management of female pelvic floor dysfunction. Int Urogynecol. 2017;28(2):191–213. doi: 10.1007/s00192-016-3123-4. - DOI - PubMed
-
- Bø K, Larsen S, Oseid S, Kvarstein B, Hagen R, Jorgensen K. Knowledge about and ability to correct pelvic floor muscle exercises in women with stress urinary incontinence. Neurourol Urodyn. 1988;69:261–262.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

