Comparison of four commercial, automated antigen tests to detect SARS-CoV-2 variants of concern
- PMID: 34415422
- PMCID: PMC8377707
- DOI: 10.1007/s00430-021-00719-0
Comparison of four commercial, automated antigen tests to detect SARS-CoV-2 variants of concern
Abstract
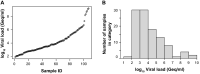
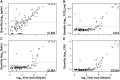
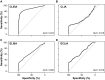
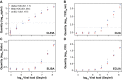
A versatile portfolio of diagnostic tests is essential for the containment of the severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) pandemic. Besides nucleic acid-based test systems and point-of-care (POCT) antigen (Ag) tests, quantitative, laboratory-based nucleocapsid Ag tests for SARS-CoV-2 have recently been launched. Here, we evaluated four commercial Ag tests on automated platforms and one POCT to detect SARS-CoV-2. We evaluated PCR-positive (n = 107) and PCR-negative (n = 303) respiratory swabs from asymptomatic and symptomatic patients at the end of the second pandemic wave in Germany (February-March 2021) as well as clinical isolates EU1 (B.1.117), variant of concern (VOC) Alpha (B.1.1.7) or Beta (B.1.351), which had been expanded in a biosafety level 3 laboratory. The specificities of automated SARS-CoV-2 Ag tests ranged between 97.0 and 99.7% (Lumipulse G SARS-CoV-2 Ag (Fujirebio): 97.03%, Elecsys SARS-CoV-2 Ag (Roche Diagnostics): 97.69%; LIAISON® SARS-CoV-2 Ag (Diasorin) and SARS-CoV-2 Ag ELISA (Euroimmun): 99.67%). In this study cohort of hospitalized patients, the clinical sensitivities of tests were low, ranging from 17.76 to 52.34%, and analytical sensitivities ranged from 420,000 to 25,000,000 Geq/ml. In comparison, the detection limit of the Roche Rapid Ag Test (RAT) was 9,300,000 Geq/ml, detecting 23.58% of respiratory samples. Receiver-operating-characteristics (ROCs) and Youden's index analyses were performed to further characterize the assays' overall performance and determine optimal assay cutoffs for sensitivity and specificity. VOCs carrying up to four amino acid mutations in nucleocapsid were detected by all five assays with characteristics comparable to non-VOCs. In summary, automated, quantitative SARS-CoV-2 Ag tests show variable performance and are not necessarily superior to a standard POCT. The efficacy of any alternative testing strategies to complement nucleic acid-based assays must be carefully evaluated by independent laboratories prior to widespread implementation.
Keywords: Automated SARS-CoV-2 antigen test; Diagnostic test; Nucleocapsid protein; Sensitivity; Specificity; VOC.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Automated antigen assays display a high heterogeneity for the detection of SARS-CoV-2 variants of concern, including several Omicron sublineages.Med Microbiol Immunol. 2023 Oct;212(5):307-322. doi: 10.1007/s00430-023-00774-9. Epub 2023 Aug 10. Med Microbiol Immunol. 2023. PMID: 37561226 Free PMC article.
-
Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2021 Mar 24;3(3):CD013705. doi: 10.1002/14651858.CD013705.pub2. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 Jul 22;7:CD013705. doi: 10.1002/14651858.CD013705.pub3. PMID: 33760236 Free PMC article. Updated.
-
Evaluation of two rapid antigen tests to detect SARS-CoV-2 in a hospital setting.Med Microbiol Immunol. 2021 Feb;210(1):65-72. doi: 10.1007/s00430-020-00698-8. Epub 2021 Jan 16. Med Microbiol Immunol. 2021. PMID: 33452927 Free PMC article.
-
Comparative evaluation of RT-PCR and antigen-based rapid diagnostic tests (Ag-RDTs) for SARS-CoV-2 detection: performance, variant specificity, and clinical implications.Microbiol Spectr. 2024 Jun 4;12(6):e0007324. doi: 10.1128/spectrum.00073-24. Epub 2024 Apr 29. Microbiol Spectr. 2024. PMID: 38683014 Free PMC article.
-
Comparison of diagnostic accuracy of rapid antigen tests for COVID-19 compared to the viral genetic test in adults: a systematic review and meta-analysis.JBI Evid Synth. 2024 Oct 1;22(10):1939-2002. doi: 10.11124/JBIES-23-00291. JBI Evid Synth. 2024. PMID: 39188132 Free PMC article.
Cited by
-
Evaluation of the DiaSorin LIAISON SARS-CoV-2 antigen assay on nasopharyngeal swabs in two different SARS-CoV-2 pandemic waves in Switzerland: The impact of the Omicron variant on its performance.J Clin Virol Plus. 2022 Aug;2(3):100095. doi: 10.1016/j.jcvp.2022.100095. Epub 2022 Jun 26. J Clin Virol Plus. 2022. PMID: 35789900 Free PMC article.
-
Reflections on COVID-19: A Literature Review of SARS-CoV-2 Testing.Vaccines (Basel). 2024 Dec 26;13(1):9. doi: 10.3390/vaccines13010009. Vaccines (Basel). 2024. PMID: 39852788 Free PMC article. Review.
-
Effective surveillance of acute COVID-19 using a cost- and labor-efficient approach: a paradigm for the longitudinal monitoring of respiratory infections in larger cohorts.Infection. 2025 May 12. doi: 10.1007/s15010-025-02526-8. Online ahead of print. Infection. 2025. PMID: 40354031
-
Variable detection of Omicron-BA.1 and -BA.2 by SARS-CoV-2 rapid antigen tests.Med Microbiol Immunol. 2023 Feb;212(1):13-23. doi: 10.1007/s00430-022-00752-7. Epub 2022 Nov 12. Med Microbiol Immunol. 2023. PMID: 36370197 Free PMC article.
-
Analytical Sensitivity of Six SARS-CoV-2 Rapid Antigen Tests for Omicron versus Delta Variant.Viruses. 2022 Mar 22;14(4):654. doi: 10.3390/v14040654. Viruses. 2022. PMID: 35458384 Free PMC article.
References
-
- Corman VM, Haage VC, Bleicker T, Schmidt ML, Mühlemann B, Zuchowski M, Jo WK, Tscheak P, Möncke-Buchner E, Müller MA, Krumbholz A, Drexler JF, Drosten C. Comparison of seven commercial SARS-CoV-2 rapid point-of-care antigen tests: a single-centre laboratory evaluation study. Lancet Microbe. 2021;2(7):e311–e319. doi: 10.1016/s2666-5247(21)00056-2. - DOI - PMC - PubMed
-
- Dinnes J, Deeks JJ, Berhane S, Taylor M, Adriano A, Davenport C, Dittrich S, Emperador D, Takwoingi Y, Cunningham J, Beese S, Domen J, Dretzke J, Ferrante di Ruffano L, Harris IM, Price MJ, Taylor-Phillips S, Hooft L, Leeflang MM, McInnes MD, Spijker R, Van den Bruel A. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2021;3(3):Cd013705. doi: 10.1002/14651858.CD013705.pub2. - DOI - PMC - PubMed
-
- Osterman A, Baldauf HM, Eletreby M, Wettengel JM, Afridi SQ, Fuchs T, Holzmann E, Maier A, Döring J, Grzimek-Koschewa N, Muenchhoff M, Protzer U, Kaderali L, Keppler OT. Evaluation of two rapid antigen tests to detect SARS-CoV-2 in a hospital setting. Med Microbiol Immunol. 2021;210(1):65–72. doi: 10.1007/s00430-020-00698-8. - DOI - PMC - PubMed
-
- Hirotsu Y, Maejima M, Shibusawa M, Nagakubo Y, Hosaka K, Amemiya K, Sueki H, Hayakawa M, Mochizuki H, Tsutsui T, Kakizaki Y, Miyashita Y, Yagi S, Kojima S, Omata M. Comparison of automated SARS-CoV-2 antigen test for COVID-19 infection with quantitative RT-PCR using 313 nasopharyngeal swabs, including from seven serially followed patients. Int J Infect Dis. 2020;99:397–402. doi: 10.1016/j.ijid.2020.08.029. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

