Capturing membrane trafficking events during 3D angiogenic development in vitro
- PMID: 34415654
- PMCID: PMC8858330
- DOI: 10.1111/micc.12726
Capturing membrane trafficking events during 3D angiogenic development in vitro
Abstract
Objectives: Vesicular trafficking dictates protein localization, functional activity, and half-life, providing a critically important regulatory step in tissue development; however, there is little information detailing endothelial-specific trafficking signatures. This is due, in part, to limitations in visualizing trafficking events in endothelial tissues. Our aim in this investigation was to explore the use of a 3-dimensional (3D) in vitro sprouting model to image endothelial membrane trafficking events.
Methods: Endothelial cells were challenged to grow sprouts in a fibrin bead assay. Thereafter, spouts were transfected with fluorescent proteins and stained for various cell markers. Sprouts were then imaged for trafficking events using live and fixed-cell microscopy.
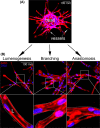
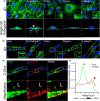
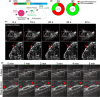
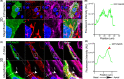
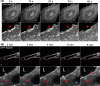
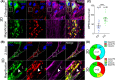
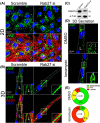
Results: Our results demonstrate that fibrin bead sprouts have a strong apicobasal polarity marked by apical localization of proteins moesin and podocalyxin. Comparison of trafficking mediators Rab27a and Rab35 between 3D sprouts and 2D culture showed that vesicular carriers can be imaged at high resolution, exhibiting proper membrane polarity solely in 3D sprouts. Lastly, we imaged exocytic events of von Willebrand Factor and demonstrated a distinct imaging advantage for monitoring secretion events in 3D sprouts as compared with 2D culture.
Conclusions: Our results establish that the fibrin bead sprouting assay is well-suited for imaging of trafficking events during angiogenic growth.
Keywords: Rab27a; Weibel-Palade body; angiogenesis; apical membrane; blood vessel; development; endothelial; endothelium; exocytosis; imaging; lumen; sprouting; trafficking; vascular; vesicle; von Willebrand factor.
© 2021 The Authors. Microcirculation published by John Wiley & Sons Ltd.
Conflict of interest statement
None.
Figures

References
-
- Kushner EJ, Bautch VL. Building blood vessels in development and disease. Curr Opin Hematol. 2013;20(3):231‐236. - PubMed
-
- Farquhar MG. Multiple pathways of exocytosis, endocytosis, and membrane recycling: validation of a Golgi route. Fed Proc. 1983;42(8):2407‐2413. - PubMed
-
- Orzech E, Cohen S, Weiss A, Aroeti B. Interactions between the exocytic and endocytic pathways in polarized Madin‐Darby canine kidney cells. J Biol Chem. 2000;275(20):15207‐15219. - PubMed
-
- Foley K, Boguslavsky S, Klip A. Endocytosis, recycling, and regulated exocytosis of glucose transporter 4. Biochemistry. 2011;50(15):3048‐3061. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

