Evaluation of drug-drug interaction potential between omecamtiv mecarbil and rosuvastatin, a BCRP substrate, with a clinical study in healthy subjects and using a physiologically-based pharmacokinetic model
- PMID: 34415673
- PMCID: PMC8604240
- DOI: 10.1111/cts.13118
Evaluation of drug-drug interaction potential between omecamtiv mecarbil and rosuvastatin, a BCRP substrate, with a clinical study in healthy subjects and using a physiologically-based pharmacokinetic model
Abstract
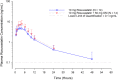
Omecamtiv mecarbil (OM) is a novel cardiac myosin activator in development for the treatment of heart failure. In vitro, OM is an inhibitor of BCRP. Rosuvastatin, a BCRP substrate, is one of the most commonly prescribed medications in patients with heart failure. The potential for a pharmacokinetic (PK) drug-drug interaction (DDI) was investigated, specifically to determine whether a single 50 mg dose of OM would impact the PKs of a single 10 mg dose of rosuvastatin in an open-label study in 14 healthy subjects. The ratios of the geometric least-square means (90% confidence intervals [CIs]) of rosuvastatin co-administered with OM compared to rosuvastatin alone were 127.1% (90% CI 113.8-141.9), 132.8% (90% CI 120.7-146.1), and 154.2% (90% CI 132.8-179.1) for area under the plasma-concentration time curve from time zero to infinity (AUCinf ), area under the plasma-concentration time curve from time zero to time of last quantifiable concentration (AUClast ), and maximum observed plasma concentration (Cmax ), respectively. Whereas the DDI study with rosuvastatin was conducted with the co-administration of a single dose of OM, in the clinical setting, patients receive OM at doses of 25, 37.5, or 50 mg twice daily (b.i.d.). Hence, to extrapolate the results of the DDI study to a clinically relevant scenario of continuous b.i.d. dosing with OM, physiologically-based pharmacokinetic (PBPK) modeling was performed to explore the potential of BCRP inhibition following continuous b.i.d. dosing of OM at the highest 50 mg dose. Modeling results indicated that following 50 mg b.i.d. dosing of OM, the predicted ratios of the geometric means (90% CIs) for rosuvastatin AUCinf and Cmax were 1.18 (90% CI 1.16-1.20) and 2.04 (90% CI 1.99-2.10), respectively. Therefore, these results suggest that OM, following multiple dose administration, is a weak inhibitor of BCRP substrates and is in accordance with that observed in the single dose OM DDI clinical study.
© 2021 Amgen Inc. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
A.T., W.S., P.K., P.J., H.Z., M.S., S.A., J.W., E.L., and S.D. are employees and shareholders of Amgen, Inc. P.K. was a full‐time employee of Amgen and worked on this assignment and is a current shareholder in Amgen. All other authors declared no competing interests for this work.
Figures
References
-
- Teerlink JR, Diaz R, Felker GM, et al. Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med. 2021;384(2):105‐116. - PubMed
-
- Teerlink JR, Diaz R, Felker GM, et al. Omecamtiv mecarbil in chronic heart failure with reduced ejection fraction: rationale and design of GALACTIC‐HF. JACC Heart Fail. 2020;8(4):329‐340. - PubMed
-
- Psotka MA, Teerlink JR. Direct myosin activation by omecamtiv mecarbil for heart failure with reduced ejection fraction. Handb Exp Pharmacol. 2017;243:465‐490. - PubMed
-
- Liu LC, Dorhout B, van der Meer P, Teerlink JR, Voors AA. Omecamtiv mecarbil: a new cardiac myosin activator for the treatment of heart failure. Expert Opin Investig Drugs. 2016;25(1):117‐127. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

