Effect on growth of exposure to maternal antiretroviral therapy in breastmilk versus extended infant nevirapine prophylaxis among HIV-exposed perinatally uninfected infants in the PROMISE randomized trial
- PMID: 34415933
- PMCID: PMC8378741
- DOI: 10.1371/journal.pone.0255250
Effect on growth of exposure to maternal antiretroviral therapy in breastmilk versus extended infant nevirapine prophylaxis among HIV-exposed perinatally uninfected infants in the PROMISE randomized trial
Abstract
Background: Malnutrition is highly prevalent in HIV-exposed perinatally uninfected infants (HEUs) increasing the risk of morbidity and mortality throughout the life course. We set out to compare the effect of postnatal exposure to maternal antiretroviral therapy (mART) in breastmilk versus infant Nevirapine prophylaxis (iNVP) on somatic growth of HEUs in the randomized PROMISE trial.
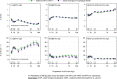
Methods and findings: We randomized 2431 mothers with HIV and their 2444 HEUs from six African countries and India 6-14 days after delivery to mART or iNVP for prevention of breastmilk HIV transmission. The mART regimen contained tenofovir/emtricitabine (99%) plus lopinavir/ritonavir. Infant growth parameters were compared at postnatal week 10, 26, 74 and 104 using World Health Organization (WHO) z-scores for length-for-age (LAZ), weight-for-age (WAZ), and head circumference-for-age (HCAZ). Week 26 LAZ was the primary endpoint measure. Student T-tests compared mean LAZ, WAZ, and HCAZ; estimated mean and 95% confidence interval (CI) are presented. Maternal and infant baseline characteristics were comparable between study arms. The estimated median breastfeeding duration was 70 weeks. After a mean follow-up of 88 weeks, mean LAZ and WAZ were below the WHO reference population mean at all timepoints, whereas mean HCAZ was not. The mART and iNVP arms did not differ for the primary outcome measure of LAZ at week 26 (p-value = 0.39; estimated mean difference (95%CI) of -0.05 (-0.18, 0.07)) or any of the other secondary growth outcome measures or timepoints (all p-values≥0.16). Secondary analyses of the primary outcome measure adjusting for week 0 LAZ and other covariates did not change these results (all p-values≥0.09). However, infants assigned to mART were more likely to have stunting compared to iNVP infants at week 26 (odds ratio (95% CI): 1.28 (1.05, 1.57)).
Conclusions: In HEUs, growth effects from postnatal exposure to mART compared to iNVP were comparable for measures on length, weight and head circumference with no clinically relevant differences between the groups. Despite breastfeeding into the second year of life, length and weight were below reference population means at all ages in both arms. Further investment is needed to optimize postnatal growth of infants born to women with HIV.
Clinical trial registration: ClinicalTrials.gov number NCT01061151.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Aizire J, Sikorskii A, Ogwang LW, Kawalazira R, Mutebe A, Familiar-Lopez I, et al.. Decreased growth among antiretroviral drug and HIV-exposed uninfected versus unexposed children in Malawi and Uganda. AIDS (London, England). 2020Feb;34(2):215–25. doi: 10.1097/QAD.0000000000002405 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UM1 AI068632/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI069530/AI/NIAID NIH HHS/United States
- UM1 AI069518/AI/NIAID NIH HHS/United States
- UM1 AI069436/AI/NIAID NIH HHS/United States
- HHSN275201800001I/HD/NICHD NIH HHS/United States
- U01 AI068632/AI/NIAID NIH HHS/United States
- HHSN275201800001C/HD/NICHD NIH HHS/United States
- UM1 AI069463/AI/NIAID NIH HHS/United States
- UM1 AI068616/AI/NIAID NIH HHS/United States
- UM1 AI106716/AI/NIAID NIH HHS/United States
- U01 AI069436/AI/NIAID NIH HHS/United States

