Impact of diagnostic strategies for tuberculosis using lateral flow urine lipoarabinomannan assay in people living with HIV
- PMID: 34416013
- PMCID: PMC8407503
- DOI: 10.1002/14651858.CD014641
Impact of diagnostic strategies for tuberculosis using lateral flow urine lipoarabinomannan assay in people living with HIV
Abstract
Background: Tuberculosis is the primary cause of hospital admission in people living with HIV, and the likelihood of death in the hospital is unacceptably high. The Alere Determine TB LAM Ag test (AlereLAM) is a point-of-care test and the only lateral flow lipoarabinomannan assay (LF-LAM) assay currently commercially available and recommended by the World Health Organization (WHO). A 2019 Cochrane Review summarised the diagnostic accuracy of LF-LAM for tuberculosis in people living with HIV. This systematic review assesses the impact of the use of LF-LAM (AlereLAM) on mortality and other patient-important outcomes.
Objectives: To assess the impact of the use of LF-LAM (AlereLAM) on mortality in adults living with HIV in inpatient and outpatient settings. To assess the impact of the use of LF-LAM (AlereLAM) on other patient-important outcomes in adults living with HIV, including time to diagnosis of tuberculosis, and time to initiation of tuberculosis treatment.
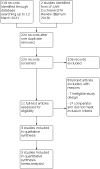
Search methods: We searched the Cochrane Infectious Diseases Group Specialized Register; the Cochrane Central Register of Controlled Trials (CENTRAL); MEDLINE (PubMed); Embase (Ovid); Science Citation Index Expanded (Web of Science), BIOSIS Previews, Scopus, LILACS; ProQuest Dissertations and Theses; ClinicalTrials.gov; and the WHO ICTRP up to 12 March 2021.
Selection criteria: Randomized controlled trials that compared a diagnostic intervention including LF-LAM with diagnostic strategies that used smear microscopy, mycobacterial culture, a nucleic acid amplification test such as Xpert MTB/RIF, or a combination of these tests. We included adults (≥ 15 years) living with HIV.
Data collection and analysis: Two review authors independently assessed trials for eligibility, extracted data, and analysed risk of bias using the Cochrane tool for assessing risk of bias in randomized studies. We contacted study authors for clarification as needed. We used risk ratio (RR) with 95% confidence intervals (CI). We used a fixed-effect model except in the presence of clinical or statistical heterogeneity, in which case we used a random-effects model. We assessed the certainty of the evidence using GRADE.
Main results: We included three trials, two in inpatient settings and one in outpatient settings. All trials were conducted in sub-Saharan Africa and assessed the impact of diagnostic strategies that included LF-LAM on mortality when the test was used in conjunction with other tuberculosis diagnostic tests or clinical assessment for clinical decision-making in adults living with HIV. Inpatient settings In inpatient settings, the use of LF-LAM testing as part of a tuberculosis diagnostic strategy likely reduces mortality in people living with HIV at eight weeks compared to routine tuberculosis diagnostic testing without LF-LAM (pooled RR 0.85, 95% CI 0.76 to 0.94; 5102 participants, 2 trials; moderate-certainty evidence). That is, people living with HIV who received LF-LAM had 15% lower risk of mortality. The absolute effect was 34 fewer deaths per 1000 (from 14 fewer to 55 fewer). In inpatient settings, the use of LF-LAM testing as part of a tuberculosis diagnostic strategy probably results in a slight increase in the proportion of people living with HIV who were started on tuberculosis treatment compared to routine tuberculosis diagnostic testing without LF-LAM (pooled RR 1.26, 95% CI 0.94 to 1.69; 5102 participants, 2 trials; moderate-certainty evidence). Outpatient settings In outpatient settings, the use of LF-LAM testing as part of a tuberculosis diagnostic strategy may reduce mortality in people living with HIV at six months compared to routine tuberculosis diagnostic testing without LF-LAM (RR 0.89, 95% CI 0.71 to 1.11; 2972 participants, 1 trial; low-certainty evidence). Although this trial did not detect a difference in mortality, the direction of effect was towards a mortality reduction, and the effect size was similar to that in inpatient settings. In outpatient settings, the use of LF-LAM testing as part of a tuberculosis diagnostic strategy may result in a large increase in the proportion of people living with HIV who were started on tuberculosis treatment compared to routine tuberculosis diagnostic testing without LF-LAM (RR 5.44, 95% CI 4.70 to 6.29, 3022 participants, 1 trial; low-certainty evidence). Other patient-important outcomes Assessment of other patient-important and implementation outcomes in the trials varied. The included trials demonstrated that a higher proportion of people living with HIV were able to produce urine compared to sputum for tuberculosis diagnostic testing; a higher proportion of people living with HIV were diagnosed with tuberculosis in the group that received LF-LAM; and the incremental diagnostic yield was higher for LF-LAM than for urine or sputum Xpert MTB/RIF.
Authors' conclusions: In inpatient settings, the use of LF-LAM as part of a tuberculosis diagnostic testing strategy likely reduces mortality and probably results in a slight increase in tuberculosis treatment initiation in people living with HIV. The reduction in mortality may be due to earlier diagnosis, which facilitates prompt treatment initiation. In outpatient settings, the use of LF-LAM testing as part of a tuberculosis diagnostic strategy may reduce mortality and may result in a large increase in tuberculosis treatment initiation in people living with HIV. Our results support the implementation of LF-LAM to be used in conjunction with other WHO-recommended tuberculosis diagnostic tests to assist in the rapid diagnosis of tuberculosis in people living with HIV.
Copyright © 2021 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
RRN has no known conflicts of interest. She serves voluntarily as Chair of a tuberculosis advocacy organisation, TB Proof, based in South Africa. Although TB Proof has not directly led advocacy efforts related to urine LAM, the organisation has supported calls to improve access to urine LAM based on WHO guidance.
PL has no known conflicts of interest.
MC has no known conflicts of interest.
SB has no known conflicts of interest.
KRS has received financial support from Cochrane Infectious Diseases, UK; McGill University, Canada; and USAID, USA, administered by the World Health Organization Global TB Programme, Switzerland for the preparation of systematic reviews and educational materials. She has also received consultancy fees from Foundation for Innovative New Diagnostics (FIND), Switzerland (for the preparation of systematic reviews and GRADE tables); honoraria; and travel support to attend WHO guideline meetings.
MS has no known conflicts of interest.
Figures



















References
References to studies included in this review
Grant 2020 {published data only}
Gupta‐Wright 2018 {published data only}
-
- Gupta-Wright A, Corbett EL, Oosterhout JJ, Wilson D, Grint D, Alufandika-Moyo M, et al. Rapid urine-based screening for tuberculosis in HIV-positive patients admitted to hospital in Africa (STAMP): a pragmatic, multicentre, parallel-group, double-blind, randomised controlled trial. Lancet 2018;392(10144):292-301. - PMC - PubMed
Peter 2016 {published data only}
-
- Peter JG, Zijenah LS, Chanda D, Clowes P, Lesosky M, Gina P, et al. Effect on mortality of point-of-care, urine-based lipoarabinomannan testing to guide tuberculosis treatment initiation in HIV-positive hospital inpatients: a pragmatic, parallel-group, multicountry, open-label, randomised controlled trial. Lancet 2016;387(10024):1187-97. - PubMed
References to studies excluded from this review
Blanc 2020 {published data only}
-
- Blanc FX, Badje AD, Bonnet M, Gabillard D, Messou E, Muzoora C, et al. Systematic or test-guided treatment for tuberculosis in HIV-Infected adults. New England Journal of Medicine 2020;382(25):2397-410. - PubMed
Cummings 2019 {published data only}
-
- Cummings MJ, Bakamutumaho B, Owor N, Kayiwa J, Byaruhanga T, Namagambo B, et al. Operational feasibility and diagnostic yield of urine TB-LAM testing among HIV-infected patients hospitalized with sepsis and septic shock in Uganda. American Journal of Respiratory and Critical Care Medicine 2019;199:2.
Gupta‐Wright 2019 {published data only}
-
- Gupta-Wright A, Corbett EL, Wilson D, Oosterhout JJ, Dheda K, Huerga H, et al. Risk score for predicting mortality including urine lipoarabinomannan detection in hospital inpatients with HIV-associated tuberculosis in sub-Saharan Africa: derivation and external validation cohort study. PLOS Medicine 2019;16(4):e1002776. - PMC - PubMed
Huerga 2019 {published data only}
-
- Huerga H, Mathabire Rucker SC, Bastard M, Dimba A, Kamba C, Amoros I, et al. Should urine-LAM tests be used in TB symptomatic HIV-positive patients when no CD4 count is available? A prospective observational cohort study from Malawi. Journal of Acquired Immune Deficifiency Syndromes 2020;83(1):24-30. - PMC - PubMed
Kubiak 2018 {published data only}
Mathabire Rucker 2019 {published data only}
Mthiyane 2019 {published data only}
Naidoo 2019 {published data only}
-
- Naidoo P, Esmail A, Peter JG, Davids M, Fadul M, Dheda K. Does the use of adjunct urine lipopolysaccharide lipoarabinomannan in HIV-infected hospitalized patients reduce the utilization of healthcare resources? A post hoc analysis of the LAM multi-country randomized controlled trial. International Journal of Infectious Diseases 2019;79:37-43. [DOI: 10.1016/j.ijid.2018.09.024] - DOI - PubMed
Additional references
Alere 2020
-
- Abbott. Alere DetermineTM TB LAM Ag product information. www.globalpointofcare.abbott/en/product-details/determine-tb-lam.html (accessed 17 December 2020).
Auld 2016
-
- Auld AF, Fielding KL, Gupta-Wright A, Lawn SD. Xpert MTB/RIF - why the lack of morbidity and mortality impact in intervention trials? Transactions of the Royal Society of Tropical Medicine and Hygiene 2016;110(8):432-44. - PubMed
Balshem 2011
-
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401-6. - PubMed
Barrera 2015
-
- Barrera E, Livchits V, Nardell E. F-A-S-T: a refocused, intensified, administrative tuberculosis transmission control strategy. International Journal of Tuberculosis and Lung Disease 2015;19(4):381-4. - PubMed
Bjerrum 2019
-
- Bjerrum S, Schiller I, Dendukuri N, Kohli M, Nathavitharana RR, Zwerling AA, et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in people living with HIV. Cochrane Database of Systematic Reviews 2019, Issue 10. Art. No: CD011420. [DOI: 10.1002/14651858.CD011420.pub3] - DOI - PMC - PubMed
Bjerrum 2020
-
- Bjerrum S, Broger T, Székely R, Mitarai S, Opintan JA, Kenu E, et al. Diagnostic accuracy of a novel and rapid lipoarabinomannan test for diagnosing tuberculosis among people with human immunodeficiency virus. Open Forum Infectious Diseases 2020;7(1):ofz530. [DOI: 10.1093/ofid/ofz530] - DOI - PMC - PubMed
Brennan 2003
-
- Brennan PJ. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis 2003;83(1-3):91-7. - PubMed
Briken 2004
-
- Briken V, Porcelli SA, Besra GS, Kremer L. Mycobacterial lipoarabinomannan and related lipoglycans: from biogenesis to modulation of the immune response. Molecular Microbiology 2004;53(2):391-403. - PubMed
Broger 2020
Calligaro 2015
-
- Calligaro GL, Theron G, Khalfey H, Peter J, Meldau R, Matinyenya B, et al. Burden of tuberculosis in intensive care units in Cape Town, South Africa, and assessment of the accuracy and effect on patient outcomes of the Xpert MTB/RIF test on tracheal aspirate samples for diagnosis of pulmonary tuberculosis: a prospective burden of disease study with a nested randomised controlled trial. Lancet Respiratory Medicine 2015;3(8):621-30. - PubMed
Churchyard 2015
-
- Churchyard GJ, Stevens WS, Mametja LD, McCarthy KM, Chihota V, Nicol MP, et al. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: a cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. Lancet Global Health 2015;3(8):e450-7. - PubMed
Covidence [Computer program]
-
- Veritas Health Innovation Covidence. Version accessed 15 March 2021. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Cox 2014
Deeks 2021
-
- Deeks JJ, Higgins JPT, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook .
di Ruffano 2012
-
- di Ruffano LF, Hyde CJ, McCaffery KJ, Bossuyt PM, Deeks JJ. Assessing the value of diagnostic tests: a framework for designing and evaluating trials. BMJ 2012;344:e686. - PubMed
Di Tanna 2019
Drain 2015
Drain 2017
-
- Drain PK, Losina E, Coleman SM, Giddy J, Ross D, Katz JN, et al. Clinic-based urinary lipoarabinomannan as a biomarker of clinical disease severity and mortality among antiretroviral therapy-naive human immunodeficiency virus-infected adults in South Africa. Open Forum Infectious Diseases 2017;4(3):ofx167. - PMC - PubMed
Ford 2016
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 20 June 2020. Available at gradepro.org.
Gupta 2015
Gupta‐Wright 2016
-
- Gupta-Wright A, Peters JA, Flach C, Lawn SD. Detection of lipoarabinomannan (LAM) in urine is an independent predictor of mortality risk in patients receiving treatment for HIV-associated tuberculosis in sub-Saharan Africa: a systematic review and meta-analysis. BMC Medicine 2016;14:53. [DOI: 10.1186/s12916-016-0603-9] - DOI - PMC - PubMed
Hanson 2017
Haraka 2021
-
- Haraka F, Kakolwa M, Schumacher SG, Nathavitharana RR, Denkinger CM, Gagneux S, et al. Impact of the diagnostic test Xpert MTB/RIF on patient outcomes for tuberculosis. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD012972. [DOI: 10.1002/14651858.CD012972.pub2] - DOI - PMC - PubMed
Higgins 2011
Hultcrantz 2017
Kaplan 2018
Karat 2017
Kay 2020
-
- Kay AW, González Fernández L, Takwoingi Y, Eisenhut M, Detjen AK, Steingart KR, et al. Xpert MTB/RIF and Xpert MTB/RIF Ultra assays for active tuberculosis and rifampicin resistance in children. Cochrane Database of Systematic Reviews 2020, Issue 8. Art. No: CD013359. [DOI: 10.1002/14651858.CD013359.pub2] - DOI - PMC - PubMed
Kohli 2021
-
- Kohli M, Schiller I, Dendukuri N, Yao M, Dheda K, Denkinger CM, et al. Xpert MTB/RIF Ultra and Xpert MTB/RIF assays for extrapulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database of Systematic Reviews 2021, Issue 1. Art. No: CD012768. [DOI: 10.1002/14651858.CD012768.pub3] - DOI - PMC - PubMed
LaCourse 2018
Lawn 2012
Lawn 2016
-
- Lawn SD, Gupta-Wright A. Detection of lipoarabinomannan (LAM) in urine is indicative of disseminated TB with renal involvement in patients living with HIV and advanced immunodeficiency: evidence and implications. Transactions of the Royal Society of Tropical Medicine and Hygiene 2016;110(3):180-5. - PMC - PubMed
Lewinsohn 2017
-
- Lewinsohn DM, Leonard MK, LoBue PA, Cohn DL, Daley CL, Desmond E, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clinical Infectious Diseases 2017;64(2):e1–33. [DOI: 10.1093/cid/ciw694] - DOI - PubMed
MacLean 2019
-
- MacLean E, Broger T, Yerlikaya S, Fernandez-Carballo BL, Pai M, Denkinger CM. A systematic review of biomarkers to detect active tuberculosis. Nature Microbiology 2019;4(5):748-58. - PubMed
Minion 2011
-
- Minion J, Leung E, Talbot E, Dheda K, Pai M, Menzies D. Diagnosing tuberculosis with urine lipoarabinomannan: systematic review and meta-analysis. European Respiratory Journal 2011;38(6):1398-405. - PubMed
Moher 2009
Mupfumi 2014
Ngwira 2019
-
- Ngwira LG, Corbett EL, Khundi M, Barnes GL, Nkhoma A, Murowa M, et al. Screening for tuberculosis with Xpert MTB/RIF versus fluorescent microscopy among adults newly diagnosed with HIV in rural Malawi: a cluster randomized trial (CHEPETSA). Clinical Infectious Diseases 2019;68(7):1176–83. [DOI: 10.1093/cid/ciy590] - DOI - PMC - PubMed
Nliwasa 2018
Pai 2016
-
- Pai M, Behr MA, Dowdy D, Dheda K, Divangahi M, Boehme CC, et al. Tuberculosis. Nature Reviews Disease Primers 2016;2:16076. - PubMed
Review Manager 2020 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Santesso 2020
-
- Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al. GRADE Working Group. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;119:126-35. [DOI: 10.1016/j.jclinepi.2019.10.014] - DOI - PubMed
Saran 2019
-
- Saran K, Masini T, Chikwanha I, Paton G, Scourse R, Kahn P, et al. Countries are out of step with international recommendations for tuberculosis testing, treatment, and care: findings from a 29-country survey of policy adoption and implementation. www.biorxiv.org/content/biorxiv/early/2019/02/01/533851.full.pdf (accessed 18 July 2019). [DOI: 10.1101/533851]
Schumacher 2016
Schumacher 2019
-
- Schumacher SG, Denkinger CM. The impact of Xpert MTB/RIF - do we have a final answer? Lancet Global Health 2019;7(2):e161-2. - PubMed
Schünemann 2016
-
- Schünemann HJ, Mustafa R, Brozek J, Santesso N, Alonso-Coello P, Guyatt G, et al, GRADE Working Group. GRADE Guidelines: 16. GRADE evidence to decision frameworks for tests in clinical practice and public health. Journal of Clinical Epidemiology 2016;76:89-98. [DOI: 10.1016/j.jclinepi.2016.01.032] - DOI - PubMed
Shah 2010
Shah 2016
-
- Shah M, Hanrahan C, Wang ZY, Dendukuri N, Lawn SD, Denkinger CM, et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in HIV-positive adults. Cochrane Database of Systematic Reviews 2016, Issue 5. Art. No: CD011420. [DOI: 10.1002/14651858.CD011420.pub2] - DOI - PMC - PubMed
Shapiro 2021
-
- Shapiro AE, Ross JM, Yao M, Schiller I, Kohli M, Dendukuri N, et al. Xpert MTB/RIF and Xpert Ultra assays for screening for pulmonary tuberculosis and rifampicin resistance in adults, irrespective of signs or symptoms. Cochrane Database of Systematic Reviews 2021, Issue 3. Art. No: CD013694. [DOI: 10.1002/14651858.CD013694.pub2] - DOI - PMC - PubMed
Shivakoti 2017
Sossen 2020
Sreeramareddy 2014
Sterne 2011
-
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;22(343):d4002. - PubMed
Theron 2014a
-
- Theron G, Zijenah L, Chanda D, Clowes P, Rachow A, Lesosky M, et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet 2014;383(9915):424-35. - PubMed
Theron 2014b
-
- Theron G, Peter J, Dowdy D, Langley I, Squire SB, Dheda K. Do high rates of empirical treatment undermine the potential effect of new diagnostic tests for tuberculosis in high-burden settings? Lancet Infectious Diseases 2014;14(6):527-32. - PubMed
Trajman 2015
WHO Compendium of WHO Guidelines 2018
-
- World Health Organization. Compendium of WHO guidelines and associated standards: ensuring optimum delivery of the cascade of care for patients with tuberculosis. Second Edition - June 2018. http://apps.who.int/iris/bitstream/handle/10665/272644/9789241514101-eng... (accessed 25 June 2021).
WHO Consolidated Guidelines (Module 3) 2020
-
- World Health Organization. WHO consolidated guidelines on tuberculosis. Module 3: diagnosis – rapid diagnostics for tuberculosis detection. Licence: CC BY-NC-SA 3.0 IGO. who.int/publications/i/item/who-consolidated-guidelines-on-tuberculosis-... (accessed 2 July 2020). - PubMed
WHO Global Tuberculosis Report 2020
-
- World Health Organization. Global Tuberculosis Report 2020. apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf (accessed 20 June 2021).
WHO Operational Handbook (Module 3) 2020
-
- World Health Organization. WHO Operational Handbook on tuberculosis. Module 3: diagnosis - rapid diagnostics for tuberculosis detection. Licence: CC BY-NC-SA 3.0 IGO. www.who.int/publications/i/item/who-operational-handbook-on-tuberculosis... (accessed 2 July 2020).
WHO Target Product Profile 2014
-
- World Health Organization. High-priority target product profiles for new tuberculosis diagnostics: report of a consensus meeting. WHO/HTM/TB/2014.18. who.int/iris/handle/10665/135617 (accessed 20 June 2020).
References to other published versions of this review
Steingart 2019
-
- Steingart KR, Nathavitharan RR, Lederer P, Chaplin M, Bjerrum S, Shah M. Impact of diagnostic strategies using lateral flow urine lipoarabinomannan assay on health outcomes for tuberculosis in people living with HIV. PROSPERO 2019 CRD42019153471. www.crd.york.ac.uk/prospero/display_record.php?RecordID=153471 (accessed 12 December 2019).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

