A microdosing framework for absolute bioavailability assessment of poorly soluble drugs: A case study on cold-labeled venetoclax, from chemistry to the clinic
- PMID: 34416076
- PMCID: PMC8742638
- DOI: 10.1111/cts.13144
A microdosing framework for absolute bioavailability assessment of poorly soluble drugs: A case study on cold-labeled venetoclax, from chemistry to the clinic
Abstract
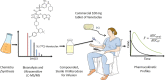
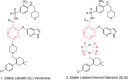
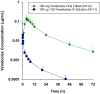
This work presents an end-to-end approach for assessing the absolute bioavailability of highly hydrophobic, poorly water-soluble compounds that exhibit high nonspecific binding using venetoclax as a model drug. The approach utilizes a stable labeled i.v. microdose and requires fewer resources compared with traditional approaches that use radioactive 14 C-labeled compounds. The stable labeled venetoclax and internal standard were synthesized, then an i.v. formulation was developed. In the clinical study, female subjects received a single oral dose of venetoclax 100 mg followed by a 100-µg i.v. dose of cold-labeled 13 C-venetoclax at the oral time of maximum concentration (Tmax ). The i.v. microdose was prepared as an extemporaneous, sterile compounded solution on the dosing day by pharmacists at the clinical site. Several measures were taken to ensure the sterility and safety of the i.v. preparation. A sensitive liquid chromatography-tandem mass spectrometry method was developed to allow the detection of plasma levels from the i.v. microdose. Plasma samples were collected through 72 h, and pharmacokinetic parameters were estimated using noncompartmental methods. Postdosing sample analysis demonstrated the consistency of the preparations and allowed the precise calculation of the pharmacokinetic parameters based on the actual injected dose. The absolute bioavailability of venetoclax was estimated at 5.4% under fasting conditions. Venetoclax extraction ratio was estimated to be 0.06 suggesting that the fraction transferred from the enterocytes into the liver is limiting venetoclax bioavailability. The proposed framework can be applied to other highly hydrophobic, poorly water-soluble compounds that exhibit high nonspecific binding to support the understanding of their absorption and disposition mechanisms and guide formulation development.
© 2021 AbbVie Inc. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
R.M., D.R., Y.L., J.B., T.E., T.G., L.R.A., and A.H.S. are AbbVie employees and may hold stock. A.A. was an AbbVie employee at the time this work was conducted and may hold stock.
Figures
References
-
- Barbhaiya RH, Dandekar KA, Greene DS. Pharmacokinetics, absolute bioavailability, and disposition of [14C]nefazodone in humans. Drug Metabol Dispos. 1996;24:91‐95. - PubMed
-
- Emami Riedmaier A, Lindley DJ, Hall JA, et al. Mechanistic physiologically based pharmacokinetic modeling of the dissolution and food effect of a biopharmaceutics classification system IV compound‐the venetoclax story. J Pharm Sci. 2018;107:495‐502. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

