Arresting vertical transmission of hepatitis B virus (AVERT-HBV) in pregnant women and their neonates in the Democratic Republic of the Congo: a feasibility study
- PMID: 34416175
- PMCID: PMC8607275
- DOI: 10.1016/S2214-109X(21)00304-1
Arresting vertical transmission of hepatitis B virus (AVERT-HBV) in pregnant women and their neonates in the Democratic Republic of the Congo: a feasibility study
Erratum in
-
Correction to Lancet Glob Health 2021; 9: 1598-607.Lancet Glob Health. 2021 Nov;9(11):e1507. doi: 10.1016/S2214-109X(21)00468-X. Lancet Glob Health. 2021. PMID: 34678194 No abstract available.
Abstract
Background: Hepatitis B virus (HBV) remains endemic throughout sub-Saharan Africa despite the widespread availability of effective childhood vaccines. In the Democratic Republic of the Congo, HBV treatment and birth-dose vaccination programmes are not established. We, therefore, aimed to evaluate the feasibility and acceptability of adding HBV testing and treatment of pregnant women as well as the birth-dose vaccination of HBV-exposed infants to the HIV prevention of mother-to-child transmission programme infrastructure in the Democratic Republic of the Congo.
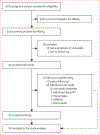
Methods: We did a feasibility study in two maternity centres in Kinshasa: Binza and Kingasani. Using the already established HIV prevention of mother-to-child transmission programme at these two maternity centres, we screened pregnant women for HBV infection at routine prenatal care registration. Those who tested positive and had a gestational age of 24 weeks or less were included in this study. Eligible pregnant women with a high viral load (≥200 000 IU/mL or HBeAg positivity, or both) were considered as having HBV of high risk of mother-to-child transmission and initiated on oral tenofovir disoproxil fumarate (300 mg/day) between 28 weeks and 32 weeks of gestation and continued through 12 weeks post partum. All HBV-exposed infants received a birth-dose of monovalent HBV vaccine (Euvax-B Pediatric: Sanofi Pasteur, Seoul, South Korea; 0·5 mL) within 24 h of life. All women were followed up for 24 weeks post partum, when they completed an exit questionnaire that assessed the acceptability of study procedures. The primary outcomes were the feasibility of screening pregnant women to identify those at high risk for HBV mother-to-child transmission and to provide them with antiviral prophylaxis, the feasibility of administrating the birth-dose vaccine to exposed infants, and the acceptability of this prevention programme. This study is registered with ClinicalTrials.gov, NCT03567382.
Findings: Between Sept 24, 2018, and Feb 22, 2019, 4016 women were approached and screened. Of these pregnant women, 109 (2·7%) were positive for HBsAg. Of the 109 women, 91 (83%) met the eligibility criteria for participation. However, only data from 90 women-excluding one woman who had a false pregnancy-were included in the study analysis. The median overall age of the enrolled women was 31 years (IQR 25-34) and the median overall gestational age was 19 weeks (15-22). Ten (11%) of 91 women evaluated had high-risk HBV infection. Nine (90%) of the ten pregnant women with high-risk HBV infection received tenofovir disoproxil fumarate and one (10%) refused therapy and withdrew from the study; five (56%) of the nine women achieved viral suppression (ie, <200 000 IU/mL) on tenofovir disoproxil fumarate therapy by the time of delivery and the remaining four (44%) had decreased viral loads from enrolment to delivery. A total of 88 infants were born to the 90 enrolled women. Of the 88 infants, 60 (68%) received a birth-dose vaccine; of these, 46 (77%) received a timely birth-dose vaccine. No cases of HBV mother-to-child transmission were observed. No serious adverse events associated with tenofovir disoproxil fumarate nor with the birth-dose vaccine were reported. Only one (11%) of nine women reported dizziness during the course of tenofovir disoproxil fumarate therapy. The study procedures were considered highly acceptable (>80%) among mothers.
Interpretation: Adding HBV screening and treatment of pregnant women and infant birth-dose vaccination to existing HIV prevention of mother-to-child transmission platforms is feasible in countries such as the Democratic Republic of the Congo. Birth-dose vaccination against HBV infection integrated within the current Expanded Programme on Immunisation and HIV prevention of mother-to-child transmission programme could accelerate progress toward HBV elimination in Africa.
Funding: Gillings Innovation Laboratory award and the National Institutes of Health.
Translations: For the French and Lingala translations of the abstract see Supplementary Materials section.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests PT and JBP report support from the American Society of Tropical Medicine and Hygiene–Burroughs Wellcome Fund awards, outside the submitted work. PT, RJ, and JBP report research support from Gilead Sciences, outside the submitted work. JBP reports grants from the US National Institutes of Health (NIH), outside the submitted work. CEM reports a grant from the Infectious Diseases Society of America, outside the submitted work. RJ reports consulting fees from Dynavax, outside the submitted work; membership on the American Association for the Study of Liver Diseases (AASLD)–Infections Diseases Society of America Hepatitis C Virus Guidelines panel and the AASLD Viral Hepatitis Elimination Task Force; and a stipend from Elsevier for editorial services as Co-Editor-in-Chief of Clinical Therapeutics. GC is an employee and shareholder of Abbott Laboratories. JBP reports research support from WHO and honoraria from Virology Education, outside the submitted work. All other authors declare no competing interests.
Figures



Comment in
-
Paving the way towards hepatitis B virus-free generations in Africa.Lancet Glob Health. 2021 Nov;9(11):e1491-e1492. doi: 10.1016/S2214-109X(21)00415-0. Lancet Glob Health. 2021. PMID: 34678185 No abstract available.
Similar articles
-
Immunoglobulin-free strategy to prevent HBV mother-to-child transmission in Cambodia (TA-PROHM): a single-arm, multicentre, phase 4 trial.Lancet Infect Dis. 2022 Aug;22(8):1181-1190. doi: 10.1016/S1473-3099(22)00206-7. Epub 2022 May 25. Lancet Infect Dis. 2022. PMID: 35643089 Clinical Trial.
-
Prevention of mother-to-child transmission of hepatitis B virus: a phase III, placebo-controlled, double-blind, randomized clinical trial to assess the efficacy and safety of a short course of tenofovir disoproxil fumarate in women with hepatitis B virus e-antigen.BMC Infect Dis. 2016 Aug 9;16:393. doi: 10.1186/s12879-016-1734-5. BMC Infect Dis. 2016. PMID: 27506549 Free PMC article. Clinical Trial.
-
Antivirals for prevention of hepatitis B virus mother-to-child transmission in human immunodeficiency virus positive pregnant women co-infected with hepatitis B virus.Cochrane Database Syst Rev. 2023 Jun 12;6(6):CD013653. doi: 10.1002/14651858.CD013653.pub2. Cochrane Database Syst Rev. 2023. PMID: 37306558 Free PMC article. Review.
-
[Clinical study on blocking mother-to-child transmission of hepatitis B virus with high viral load and HBeAg positivity during pregnancy in Guizhou province].Zhonghua Gan Zang Bing Za Zhi. 2018 Dec 20;26(12):945-950. doi: 10.3760/cma.j.issn.1007-3418.2018.12.013. Zhonghua Gan Zang Bing Za Zhi. 2018. PMID: 30669789 Chinese.
-
Efficacy and safety of antiviral prophylaxis during pregnancy to prevent mother-to-child transmission of hepatitis B virus: a systematic review and meta-analysis.Lancet Infect Dis. 2021 Jan;21(1):70-84. doi: 10.1016/S1473-3099(20)30586-7. Epub 2020 Aug 14. Lancet Infect Dis. 2021. PMID: 32805200
Cited by
-
Epidemiology of hepatitis B virus infection among pregnant women in Africa: a systematic review and meta-analysis.BMC Infect Dis. 2024 Sep 5;24(1):921. doi: 10.1186/s12879-024-09839-3. BMC Infect Dis. 2024. PMID: 39237884 Free PMC article.
-
Trends in disease burden of hepatitis B infection in Jiangsu Province, China, 1990-2021.Infect Dis Model. 2023 Jul 10;8(3):832-841. doi: 10.1016/j.idm.2023.07.007. eCollection 2023 Sep. Infect Dis Model. 2023. PMID: 37520113 Free PMC article.
-
Enhancing interventions for prevention of mother-to-child- transmission of hepatitis B virus.JHEP Rep. 2023 Apr 24;5(8):100777. doi: 10.1016/j.jhepr.2023.100777. eCollection 2023 Aug. JHEP Rep. 2023. PMID: 37554925 Free PMC article. Review.
-
Intra-familial transmission of Hepatitis B virus in a peri-urban community from the Democratic Republic of the Congo.Trop Med Health. 2025 Jul 28;53(1):99. doi: 10.1186/s41182-025-00781-x. Trop Med Health. 2025. PMID: 40721823 Free PMC article.
-
Rapid Diagnostic Test for Hepatitis B Virus Viral Load Based on Recombinase Polymerase Amplification Combined with a Lateral Flow Read-Out.Diagnostics (Basel). 2022 Mar 2;12(3):621. doi: 10.3390/diagnostics12030621. Diagnostics (Basel). 2022. PMID: 35328174 Free PMC article.
References
-
- WHO. Global hepatitis report, 2017. Geneva: World Health Organization, 2017. https://www.who.int/publications/i/item/global-hepatitis-report-2017 (accessed July 21, 2020).
-
- Gentile I, Zappulo E, Buonomo AR, Borgia G. Prevention of mother-to-child transmission of hepatitis B virus and hepatitis C virus. Expert Rev Anti Infect Ther 2014; 12: 775–82. - PubMed
-
- WHO. Prevention of mother-to-child transmission of hepatitis B virus: guidelines on antiviral prophylaxis in pregnancy. Geneva: World Health Organization, 2020. https://www.who.int/publications/i/item/978-92-4-000270-8 (accessed May 25, 2021). - PubMed
-
- Zou H, Chen Y, Duan Z, Zhang H, Pan C. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAg-positive mothers. J Viral Hepat 2012; 19: e18–25. - PubMed
-
- Schillie S, Walker T, Veselsky S, et al. Outcomes of infants born to women infected with hepatitis B. Pediatrics 2015; 135: e1141–47. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

