The long-term sequelae of COVID-19: an international consensus on research priorities for patients with pre-existing and new-onset airways disease
- PMID: 34416191
- PMCID: PMC8372501
- DOI: 10.1016/S2213-2600(21)00286-1
The long-term sequelae of COVID-19: an international consensus on research priorities for patients with pre-existing and new-onset airways disease
Abstract
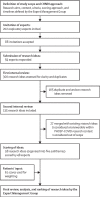
Persistent ill health after acute COVID-19-referred to as long COVID, the post-acute COVID-19 syndrome, or the post-COVID-19 condition-has emerged as a major concern. We undertook an international consensus exercise to identify research priorities with the aim of understanding the long-term effects of acute COVID-19, with a focus on people with pre-existing airways disease and the occurrence of new-onset airways disease and associated symptoms. 202 international experts were invited to submit a minimum of three research ideas. After a two-phase internal review process, a final list of 98 research topics was scored by 48 experts. Patients with pre-existing or post-COVID-19 airways disease contributed to the exercise by weighting selected criteria. The highest-ranked research idea focused on investigation of the relationship between prognostic scores at hospital admission and morbidity at 3 months and 12 months after hospital discharge in patients with and without pre-existing airways disease. High priority was also assigned to comparisons of the prevalence and severity of post-COVID-19 fatigue, sarcopenia, anxiety, depression, and risk of future cardiovascular complications in patients with and without pre-existing airways disease. Our approach has enabled development of a set of priorities that could inform future research studies and funding decisions. This prioritisation process could also be adapted to other, non-respiratory aspects of long COVID.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests CEB reports grants from the Post-hospitalisation COVID-19 study during the conduct of the study. JRH reports personal fees and non-financial support from pharmaceutical companies (AstraZeneca and GlaxoSmithKline) that make medicines to treat chronic obstructive pulmonary disease, outside the submitted work. JDC reports grants and personal fees from AstraZeneca and Boehringer Ingelheim, and personal fees from GlaxoSmithKline, Insmed, Novartis, Chiesi, and Zambon, outside the submitted work. PEP reports personal fees and non-financial support from AstraZeneca, and a grant and non-financial support from GlaxoSmithKline, outside the submitted work. AS reports grants from the Health Data Research UK BREATHE Hub, the Medical Research Council, and the National Institute for Health Research, during the conduct of the study. ADS has received medical education grant support for BRONCH-UK, a UK bronchiectasis network, from GlaxoSmithKline, Gilead, Chiesi, and Forest labs, and his institution receives fees for his work as coordinating investigator on a phase 3 trial in bronchiectasis sponsored by Bayer. All other authors declare no competing interests.
Figures
Comment in
-
Charting a course for the management of long COVID.Lancet Respir Med. 2021 Dec;9(12):1358-1360. doi: 10.1016/S2213-2600(21)00314-3. Epub 2021 Aug 17. Lancet Respir Med. 2021. PMID: 34416190 Free PMC article. No abstract available.
References
-
- World Health Organization WHO Coronavirus disease dashboard. https://covid19.who.int/
-
- Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat Med. 2021;27:28–33. - PubMed