Clinical follow-up practices after cervical cancer screening by co-testing: A population-based study of adherence to U.S. guideline recommendations
- PMID: 34416221
- PMCID: PMC8595756
- DOI: 10.1016/j.ypmed.2021.106770
Clinical follow-up practices after cervical cancer screening by co-testing: A population-based study of adherence to U.S. guideline recommendations
Abstract
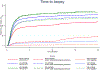
Failure to follow-up women after abnormal cervical screening could lead to cervical cancers, yet little is known about adherence to recommended follow-up after abnormal co-testing [cytology and high-risk human papillomavirus (hrHPV) testing]. We documented clinical management following cervical screening by co-testing in a diverse population-based setting. A statewide surveillance program for cervical screening, diagnosis, and treatment was used to investigate all cytology, hrHPV and biopsy reports in the state of New Mexico from January 2015 through August 2019. Guideline-adherent follow-up after co-testing required 1) biopsy within 6 months for low-grade cytology if positive for hrHPV, for high-grade cytology irrespective of hrHPV, and for HPV 16/18 positive results irrespective of cytology and; 2) repeat co-testing within 18 months if cytology was negative and hrHPV test was positive (excluding types 16/18). Screening co-tests (2015-2017) for 164,522 women were analyzed using descriptive statistics, Kaplan Meier curves, and pairwise comparisons between groups. Guideline adherence was highest when both cytology and hrHPV tests were abnormal, ranging from 61.7% to 80.3%. Guideline-adherent follow-up was lower for discordant results. Women with high-grade cytology were less likely to receive a timely biopsy when hrHPV-testing was negative (48.1%) versus positive (83.3%) (p < 0.001). Only 47.9% of women received biopsies following detection of HPV16/18 with normal cytology, and 30.8% received no follow-up within 18-months. Among women with hrHPV-positive normal cytology without evidence of HPV 16/18 infection, 51% received no follow-up within 18 months. Provider education and creation of robust recall systems may help ensure appropriate follow-up of abnormal screening results.
Keywords: Cervical câncer screening; Clinical practice; Co-testing; Cotesting follow-up.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- ACOG, 2013. Don’t Perform Routine Annual Cervical Cytology Screening (Pap Tests) in women 30–65 Years of Age. Accessed December 18, 2019. http://www.choosingwisely.org/clinician-lists/american-college-obstetric....
-
- Baron RC, Melillo S, Rimer BK, et al., 2010. Intervention to increase recommendation and delivery of screening for breast, cervical, and colorectal cancers by healthcare providers a systematic review of provider reminders. Am. J. Prev. Med. 38 (1), 110–117. 10.1016/j.amepre.2009.09.031. - DOI - PubMed

