Stem-loop binding protein and metal carcinogenesis
- PMID: 34416372
- PMCID: PMC8627438
- DOI: 10.1016/j.semcancer.2021.08.006
Stem-loop binding protein and metal carcinogenesis
Abstract
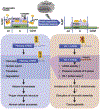
Pre-mRNA processing of the replication-dependent canonical histone mRNAs requires an endonucleolytic cleavage immediately after a conserved stem loop structure which occurs before RNA Pol II encounters any poly(A) signal. Thus, in contrast to all other eukaryotic mRNAs, the canonical histone mRNAs are not polyadenylated in their 3' ends. The binding of stem-loop binding protein (SLBP) to the stem loop structure of the histone mRNAs is required for this process. SLBP is also involved in regulation of histone mRNA nuclear export, degradation, and translation. Depletion of SLBP has been shown to induce polyadenylation of histone mRNAs and alteration of histone protein levels, which are considered to contribute to the observed aberrant cell cycle progress and genomic instability resulting from the loss of SLBP function. Recent studies have demonstrated that some heavy metal carcinogens, including arsenic and nickel, can induce the loss of SLBP and the gain of polyadenylation of canonical histone mRNAs. Polyadenylated canonical histone H3 can result in abnormal transcription, cell cycle arrest, genomic instability, and cell transformation, which links SLBP depletion and subsequent histone mRNA misprocessing to cancer. This review seeks to briefly summarize what is known about regulation of SLBP expression, consequences of SLBP depletion, its roles in cancer-related end points, with particular focus on metal-induced SLBP depletion and the potential of SLBP depletion as a new mechanism for metal-induced carcinogenesis.
Keywords: Carcinogenesis; Heavy metal; Histone; Polyadenylation; Stem-loop binding protein.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of Interest
The authors declare that there are no conflicts of interest.
Figures
References
-
- Hanson RJ, Sun J, Willis DG, Marzluff WF, Efficient extraction and partial purification of the polyribosome-associated stem-loop binding protein bound to the 3’ end of histone mRNA, Biochemistry 35(7) (1996) 2146–56. - PubMed
-
- Wang ZF, Whitfield ML, Ingledue TC 3rd, Dominski Z, Marzluff WF, The protein that binds the 3’ end of histone mRNA: a novel RNA-binding protein required for histone pre-mRNA processing, Genes Dev 10(23) (1996) 3028–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

