Murine BST2/tetherin promotes measles virus infection of neurons
- PMID: 34416448
- PMCID: PMC8491112
- DOI: 10.1016/j.virol.2021.08.005
Murine BST2/tetherin promotes measles virus infection of neurons
Abstract
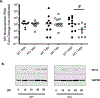
BST2/tetherin is a transmembrane protein with antiviral activity; it is synthesized following exposure to interferons, and restricts the release of budding virus particles by tethering them to the host cell membrane. We previously showed that BST2 is induced in primary neurons following measles virus (MV) infection or type I interferon; however, BST2 was dispensable for protection against challenge with neuron-restricted MV. Here, we define the contribution of BST-2 in neuronal MV infection. Surprisingly, and in contrast to its antiviral role in non-neuronal cells, murine BST2 promotes MV infection in brains of permissive mice and in primary neuron cultures. Moreover, BST2 expression was predominantly observed in the non-synaptic fraction of purified neurons. These studies highlight a cell-type dependent role of a well-characterized antiviral protein in enhancing neuronal infection.
Keywords: BST2; Measles virus; Neuron; Tetherin.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest
The authors declare no conflicts of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone Marrow Stromal Cell Antigen 2 Is a Specific Marker of Type I IFN-Producing Cells in the Naive Mouse, but a Promiscuous Cell Surface Antigen following IFN Stimulation. Journal of Immunology. 2006;177(5):3260–3265. - PubMed
-
- Goto T, Kennel SJ, Abe M, et al.A Novel Membrane Antigen Selectively Expressed on Terminally Differentiated Human B Cells. Blood. 1994;84:1922–1930. - PubMed
-
- Neil SJD, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451(7177):425–430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

