The long and winding road: human papillomavirus entry and subcellular trafficking
- PMID: 34416595
- PMCID: PMC8500947
- DOI: 10.1016/j.coviro.2021.07.010
The long and winding road: human papillomavirus entry and subcellular trafficking
Abstract
Human papillomaviruses (HPVs) infect and replicate in differentiating mucosal and cutaneous epithelium. Most HPV infections are asymptomatic or cause transient benign neoplasia. However, persistent infections by oncogenic HPV types can progress to cancer. During infectious entry into host keratinocytes, HPV particles interact with many host proteins, beginning with major capsid protein L1 binding to cellular heparan sulfate and a series of enzymatic capsid modifications that promote infectious cellular entry. After utilizing the endosomal pathway to uncoat the viral genome (vDNA), the minor capsid protein L2/vDNA complex is retrograde trafficked to the Golgi, and thereafter, to the nucleus where viral transcription initiates. Post-Golgi trafficking is dependent on mitosis, with L2-dependent tethering of vDNA to mitotic chromosomes before accumulation at nuclear substructures in G1. This review summarizes the current knowledge of the HPV entry pathway, the role of cellular proteins in this process, and notes many gaps in our understanding.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Competing interests
The authors declare that this review was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
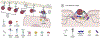

Similar articles
-
Vesicular trafficking permits evasion of cGAS/STING surveillance during initial human papillomavirus infection.PLoS Pathog. 2020 Nov 30;16(11):e1009028. doi: 10.1371/journal.ppat.1009028. eCollection 2020 Nov. PLoS Pathog. 2020. PMID: 33253291 Free PMC article.
-
Subcellular Trafficking of the Papillomavirus Genome during Initial Infection: The Remarkable Abilities of Minor Capsid Protein L2.Viruses. 2017 Dec 3;9(12):370. doi: 10.3390/v9120370. Viruses. 2017. PMID: 29207511 Free PMC article. Review.
-
Translocation of the papillomavirus L2/vDNA complex across the limiting membrane requires the onset of mitosis.PLoS Pathog. 2017 May 2;13(5):e1006200. doi: 10.1371/journal.ppat.1006200. eCollection 2017 May. PLoS Pathog. 2017. PMID: 28463988 Free PMC article.
-
HPV16 entry requires dynein for minus-end transport and utilizes kinesin Kif11 for plus-end transport along microtubules during mitosis.J Virol. 2025 Jan 31;99(1):e0093724. doi: 10.1128/jvi.00937-24. Epub 2024 Dec 4. J Virol. 2025. PMID: 39629998 Free PMC article.
-
Host-cell factors involved in papillomavirus entry.Med Microbiol Immunol. 2012 Nov;201(4):437-48. doi: 10.1007/s00430-012-0270-1. Epub 2012 Sep 13. Med Microbiol Immunol. 2012. PMID: 22972234 Review.
Cited by
-
Chondroitin Sulfate Proteoglycans Are De Facto Cellular Receptors for Human Papillomavirus 16 under High Serum Conditions.J Virol. 2022 Apr 13;96(7):e0185721. doi: 10.1128/jvi.01857-21. Epub 2022 Mar 14. J Virol. 2022. PMID: 35285688 Free PMC article.
-
High-Risk Human Papillomavirus and Epstein-Barr Virus Coinfection: A Potential Role in Head and Neck Carcinogenesis.Biology (Basel). 2021 Nov 26;10(12):1232. doi: 10.3390/biology10121232. Biology (Basel). 2021. PMID: 34943147 Free PMC article. Review.
-
HPV and Cervical Cancer: Molecular and Immunological Aspects, Epidemiology and Effect of Vaccination in Latin American Women.Viruses. 2024 Feb 21;16(3):327. doi: 10.3390/v16030327. Viruses. 2024. PMID: 38543693 Free PMC article. Review.
-
Papillomavirus-like particles as vectors for ex vivo gene therapy of the skin.Mol Ther Nucleic Acids. 2025 Mar 5;36(2):102501. doi: 10.1016/j.omtn.2025.102501. eCollection 2025 Jun 10. Mol Ther Nucleic Acids. 2025. PMID: 40171279 Free PMC article.
-
Global transcriptomic network analysis of the crosstalk between microbiota and cancer-related cells in the oral-gut-lung axis.Front Cell Infect Microbiol. 2024 Aug 20;14:1425388. doi: 10.3389/fcimb.2024.1425388. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39228892 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

