Impact of the First Generation of Children's Oncology Group Clinical Trials on Clinical Practice for Wilms Tumor
- PMID: 34416705
- PMCID: PMC8805512
- DOI: 10.6004/jnccn.2021.7070
Impact of the First Generation of Children's Oncology Group Clinical Trials on Clinical Practice for Wilms Tumor
Abstract
Refinements in surgery, radiation therapy, and chemotherapy since the mid-20th century have resulted in a survival rate exceeding 90% for patients with Wilms tumor (WT). Although this figure is remarkable, a significant proportion of patients continue to have event-free survival (EFS) estimates of <75%, and nearly 25% of survivors experience severe chronic medical conditions. The first-generation Children's Oncology Group (COG) renal tumor trials (AREN '0'), which opened to enrollment in 2006, focused on augmenting treatment regimens for WT subgroups with predicted EFS <75% to 80%, including those with the adverse prognostic marker of combined loss of heterozygosity (LOH) at chromosomes 1p/16q, pulmonary metastasis with incomplete lung nodule response after 6 weeks of chemotherapy, bilateral disease, and anaplastic histology. Conversely, therapy was reduced for patient subgroups with good outcomes and potential for long-term toxicity, such as those with lung metastasis with complete lung nodule response after 6 weeks of chemotherapy. This article summarizes the key findings of the first-generation COG renal tumor studies and their implications for clinical practice.
Figures

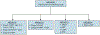

Comment in
-
Letter to the Editor: Impact of the First Generation of Children's Oncology Group Clinical Trials on Clinical Practice for Wilms Tumor.J Natl Compr Canc Netw. 2022 Mar;20(3):xlvi-xlvii. doi: 10.6004/jnccn.2021.7109. J Natl Compr Canc Netw. 2022. PMID: 35276668 No abstract available.
-
Authors' Reply to the Letter to the Editor by Daniel M. Green.J Natl Compr Canc Netw. 2022 Mar;20(3):xlvii-xlviii. doi: 10.6004/jnccn.2022.7002. J Natl Compr Canc Netw. 2022. PMID: 35276672 No abstract available.
References
-
- Nakayama DK, Bonasso PC. The history of multimodal treatment of Wilms’ tumor. Am Surg 2016;82:487–492. - PubMed
-
- Grundy PE, Breslow NE, Li S, et al. Loss of heterozygosity for chromosomes 1p and 16q is an adverse prognostic factor in favorable-histology Wilms tumor: a report from the National Wilms Tumor Study Group. J Clin Oncol 2005;23:7312–7321. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

