Single-cell transcriptomics reveals lasting changes in the lung cellular landscape into adulthood after neonatal hyperoxic exposure
- PMID: 34417156
- PMCID: PMC8710996
- DOI: 10.1016/j.redox.2021.102091
Single-cell transcriptomics reveals lasting changes in the lung cellular landscape into adulthood after neonatal hyperoxic exposure
Abstract
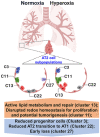
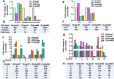
Ventilatory support, such as supplemental oxygen, used to save premature infants impairs the growth of the pulmonary microvasculature and distal alveoli, leading to bronchopulmonary dysplasia (BPD). Although lung cellular composition changes with exposure to hyperoxia in neonatal mice, most human BPD survivors are weaned off oxygen within the first weeks to months of life, yet they may have persistent lung injury and pulmonary dysfunction as adults. We hypothesized that early-life hyperoxia alters the cellular landscape in later life and predicts long-term lung injury. Using single-cell RNA sequencing, we mapped lung cell subpopulations at postnatal day (pnd)7 and pnd60 in mice exposed to hyperoxia (95% O2) for 3 days as neonates. We interrogated over 10,000 cells and identified a total of 45 clusters within 32 cell states. Neonatal hyperoxia caused persistent compositional changes in later life (pnd60) in all five type II cell states with unique signatures and function. Premature infants requiring mechanical ventilation with different durations also showed similar alterations in these unique signatures of type II cell states. Pathologically, neonatal hyperoxic exposure caused alveolar simplification in adult mice. We conclude that neonatal hyperoxia alters the lung cellular landscape in later life, uncovering neonatal programing of adult lung dysfunction.
Keywords: Alveolar type I and Alveolar type II cells; Bronchopulmonary dysplasia; Hyperoxic lung injury; Lipid and matrix homeostasis; Progenitor cells.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


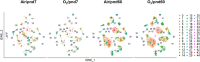





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

