Effectiveness of BNT162b2 and mRNA-1273 covid-19 vaccines against symptomatic SARS-CoV-2 infection and severe covid-19 outcomes in Ontario, Canada: test negative design study
- PMID: 34417165
- PMCID: PMC8377789
- DOI: 10.1136/bmj.n1943
Effectiveness of BNT162b2 and mRNA-1273 covid-19 vaccines against symptomatic SARS-CoV-2 infection and severe covid-19 outcomes in Ontario, Canada: test negative design study
Abstract
Objective: To estimate the effectiveness of mRNA covid-19 vaccines against symptomatic infection and severe outcomes (hospital admission or death).
Design: Test negative design study.
Setting: Ontario, Canada between 14 December 2020 and 19 April 2021.
Participants: 324 033 community dwelling people aged ≥16 years who had symptoms of covid-19 and were tested for SARS-CoV-2.
Interventions: BNT162b2 (Pfizer-BioNTech) or mRNA-1273 (Moderna) vaccine.
Main outcome measures: Laboratory confirmed SARS-CoV-2 by reverse transcription polymerase chain reaction (RT-PCR) and hospital admissions and deaths associated with SARS-CoV-2 infection. Multivariable logistic regression was adjusted for personal and clinical characteristics associated with SARS-CoV-2 and vaccine receipt to estimate vaccine effectiveness against symptomatic infection and severe outcomes.
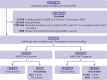
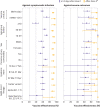
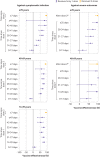
Results: Of 324 033 people with symptoms, 53 270 (16.4%) were positive for SARS-CoV-2 and 21 272 (6.6%) received at least one dose of vaccine. Among participants who tested positive, 2479 (4.7%) were admitted to hospital or died. Vaccine effectiveness against symptomatic infection observed ≥14 days after one dose was 60% (95% confidence interval 57% to 64%), increasing from 48% (41% to 54%) at 14-20 days after one dose to 71% (63% to 78%) at 35-41 days. Vaccine effectiveness observed ≥7 days after two doses was 91% (89% to 93%). Vaccine effectiveness against hospital admission or death observed ≥14 days after one dose was 70% (60% to 77%), increasing from 62% (44% to 75%) at 14-20 days to 91% (73% to 97%) at ≥35 days, whereas vaccine effectiveness observed ≥7 days after two doses was 98% (88% to 100%). For adults aged ≥70 years, vaccine effectiveness estimates were observed to be lower for intervals shortly after one dose but were comparable to those for younger people for all intervals after 28 days. After two doses, high vaccine effectiveness was observed against variants with the E484K mutation.
Conclusions: Two doses of mRNA covid-19 vaccines were observed to be highly effective against symptomatic infection and severe outcomes. Vaccine effectiveness of one dose was observed to be lower, particularly for older adults shortly after the first dose.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the Public Health Agency of Canada, the Canadian Institutes of Health Research, and Ontario’s Ministry of Health and Ministry of Long term Care for the submitted work. KW is chief executive officer of CANImmunize and serves on the data safety board for the Medicago covid-19 vaccine trial. SMM has received unrestricted research grants from Merck, GlaxoSmithKline, Sanofi Pasteur, Pfizer, and Roche-Assurex for unrelated studies. SMM has received fees as an advisory board member for GlaxoSmithKline, Merck, Pfizer, Sanofi Pasteur, and Seqirus. CHR has received an unrestricted research grant from Pfizer for an unrelated study.
Figures


References
-
- National Advisory Committee on Immunization. COVID-19 vaccine: Guidance on the prioritization of initial doses - Canada.ca. 2021. https://www.canada.ca/en/public-health/services/immunization/national-advisory-c... (accessed 2 May 2021).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
