Regulation of metamorphosis in neopteran insects is conserved in the paleopteran Cloeon dipterum (Ephemeroptera)
- PMID: 34417295
- PMCID: PMC8403931
- DOI: 10.1073/pnas.2105272118
Regulation of metamorphosis in neopteran insects is conserved in the paleopteran Cloeon dipterum (Ephemeroptera)
Abstract
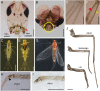
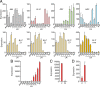
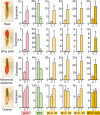
In the Paleozoic era, more than 400 Ma, a number of insect groups continued molting after forming functional wings. Today, however, flying insects stop molting after metamorphosis when they become fully winged. The only exception is the mayflies (Paleoptera, Ephemeroptera), which molt in the subimago, a flying stage between the nymph and the adult. However, the identity and homology of the subimago still is underexplored. Debate remains regarding whether this stage represents a modified nymph, an adult, or a pupa like that of butterflies. Another relevant question is why mayflies have the subimago stage despite the risk of molting fragile membranous wings. These questions have intrigued numerous authors, but nonetheless, clear answers have not yet been found. By combining morphological studies, hormonal treatments, and molecular analysis in the mayfly Cloeon dipterum, we found answers to these old questions. We observed that treatment with a juvenile hormone analog in the last nymphal instar stimulated the expression of the Kr-h1 gene and reduced that of E93, which suppress and trigger metamorphosis, respectively. The regulation of metamorphosis thus follows the MEKRE93 pathway, as in neopteran insects. Moreover, the treatment prevented the formation of the subimago. These findings suggest that the subimago must be considered an instar of the adult mayfly. We also observed that the forelegs dramatically grow between the last nymphal instar, the subimago, and the adult. This necessary growth spread over the last two stages could explain, at least in part, the adaptive sense of the subimago.
Keywords: insect development; insect endocrinology; insect evolution; insect metamorphosis.
Conflict of interest statement
The authors declare no competing interest.
Figures


Comment in
-
Mayfly metamorphosis: Adult winged insects that molt.Proc Natl Acad Sci U S A. 2021 Sep 21;118(38):e2114128118. doi: 10.1073/pnas.2114128118. Proc Natl Acad Sci U S A. 2021. PMID: 34521757 Free PMC article. No abstract available.
References
-
- Belles X., Insect Metamorphosis. From Natural History to Regulation of Development and Evolution (Academic Press, 2020).
-
- Kukalová-Peck J., Origin of the insect wing and wing articulation from the arthropodan leg. Can. J. Zool. 61, 1618–1669 (1983).
-
- Kukalová-Peck J., “Fossil history and the evolution of hexapod structures” in The Insects of Australia, Naumann I. D., Ed. (Melbourne University Press, 1991), pp. 141–179.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

