COVID-19 Vaccination Associated With Reduced Postoperative SARS-CoV-2 Infection and Morbidity
- PMID: 34417362
- PMCID: PMC8678152
- DOI: 10.1097/SLA.0000000000005176
COVID-19 Vaccination Associated With Reduced Postoperative SARS-CoV-2 Infection and Morbidity
Abstract
Objective: The purpose of this study was to determine the effect of COVID-19 vaccination on postoperative mortality, pulmonary and thrombotic complications, readmissions and hospital lengths of stay among patients undergoing surgery in the United States.
Background: While vaccination prevents COVID-19, little is known about its impact on postoperative complications.
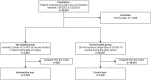
Methods: This is a nationwide observational cohort study of all 1,255 Veterans Affairs facilities nationwide. We compared patients undergoing surgery at least 2 weeks after their second dose of the Pfizer BioNTech or Moderna vaccines, to contemporary propensity score matched controls. Primary endpoints were 30-day mortality and postoperative COVID-19 infection. Secondary endpoints were pulmonary or thrombotic complications, readmissions, and hospital lengths of stay.
Results: 30,681 patients met inclusion criteria. After matching, there were 3,104 in the vaccination group (1,903 received the Pfizer BioNTech, and 1,201 received the Moderna vaccine) and 7,438 controls. Full COVID-19 vaccination was associated with lower rates of postoperative 30-day COVID-19 infection (Incidence Rate Ratio and 95% confidence intervals, 0.09 [0.01,0.44]), pulmonary complications (0.54 [0.39, 0.72]), thrombotic complications (0.68 [0.46, 0.99]) and decreased hospital lengths of stay (0.78 [0.69, 0.89]). Complications were also low in vaccinated patients who tested COVID-19 positive before surgery but events were too few to detect a significant difference compared to controls.
Conclusion: COVID-19 vaccination is associated with lower rates of postoperative morbidity. The benefit is most pronounced among individuals who have never had a COVID-19 infection before surgery.
Copyright © 2021 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
The authors report no conflicts of interest.
Figures
References
-
- Palacios R, Patiño EG, de Oliveira Piorelli R, et al. . Double-Blind, Randomized, Placebo-Controlled Phase III Clinical Trial to Evaluate the Efficacy and Safety of treating Healthcare Professionals with the Adsorbed COVID-19 (Inactivated) Vaccine Manufactured by Sinovac - PROFISCOV: A structured summary of a study protocol for a randomised controlled trial. Trials 2020; 21:853. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous