Extracellular fluid, cerebrospinal fluid and plasma biomarkers of axonal and neuronal injury following intracerebral hemorrhage
- PMID: 34417515
- PMCID: PMC8379247
- DOI: 10.1038/s41598-021-96364-x
Extracellular fluid, cerebrospinal fluid and plasma biomarkers of axonal and neuronal injury following intracerebral hemorrhage
Abstract
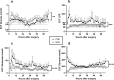
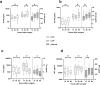
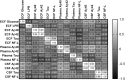
Spontaneous intracerebral hemorrhage (ICH) is the most devastating form of stroke. To refine treatments, improved understanding of the secondary injury processes is needed. We compared energy metabolic, amyloid and neuroaxonal injury biomarkers in extracellular fluid (ECF) from the perihemorrhagic zone (PHZ) and non-injured (NCX) brain tissue, cerebrospinal fluid (CSF) and plasma. Patients (n = 11; age 61 ± 10 years) undergoing ICH surgery received two microdialysis (MD) catheters, one in PHZ, and one in NCX. ECF was analysed at three time intervals within the first 60 h post- surgery, as were CSF and plasma samples. Amyloid-beta (Aβ) 40 and 42, microtubule associated protein tau (tau), and neurofilament-light (NF-L) were analysed using Single molecule array (Simoa) technology. Median biomarker concentrations were lowest in plasma, higher in ECF and highest in CSF. Biomarker levels varied over time, with different dynamics in the three fluid compartments. In the PHZ, ECF levels of Aβ40 were lower, and tau higher when compared to the NCX. Altered levels of Aβ peptides, NF-L and tau may reflect brain tissue injury following ICH surgery. However, the dynamics of biomarker levels in the different fluid compartments should be considered in the study of pathophysiology or biomarkers in ICH patients.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Effects of time of the day at sampling on CSF and plasma levels of Alzheimer' disease biomarkers.Alzheimers Res Ther. 2024 Jun 22;16(1):132. doi: 10.1186/s13195-024-01503-x. Alzheimers Res Ther. 2024. PMID: 38909218 Free PMC article.
-
Plasma biomarkers of amyloid, tau, axonal, and neuroinflammation pathologies in dementia with Lewy bodies.Alzheimers Res Ther. 2024 Jul 3;16(1):146. doi: 10.1186/s13195-024-01502-y. Alzheimers Res Ther. 2024. PMID: 38961441 Free PMC article.
-
Time Trends of Cerebrospinal Fluid Biomarkers of Neurodegeneration in Idiopathic Normal Pressure Hydrocephalus.J Alzheimers Dis. 2021;80(4):1629-1642. doi: 10.3233/JAD-201361. J Alzheimers Dis. 2021. PMID: 33720890 Free PMC article.
-
CSF and blood levels of Neurofilaments, T-Tau, P-Tau, and Abeta-42 in amyotrophic lateral sclerosis: a systematic review and meta-analysis.J Transl Med. 2024 Oct 21;22(1):953. doi: 10.1186/s12967-024-05767-7. J Transl Med. 2024. PMID: 39434139 Free PMC article.
-
CSF and plasma biomarkers in cerebral amyloid angiopathy: A single-center study and a systematic review/meta-analysis.Eur Stroke J. 2025 Mar;10(1):278-288. doi: 10.1177/23969873241260538. Epub 2024 Jun 13. Eur Stroke J. 2025. PMID: 38869035 Free PMC article.
Cited by
-
Deciphering the role of miRNA-mRNA interactions in cerebral vasospasm post intracranial hemorrhage.Front Mol Biosci. 2025 Feb 6;12:1492729. doi: 10.3389/fmolb.2025.1492729. eCollection 2025. Front Mol Biosci. 2025. PMID: 39981435 Free PMC article.
-
A Minimally-Invasive Method for Serial Cerebrospinal Fluid Collection and Injection in Rodents with High Survival Rates.Biomedicines. 2023 Jun 1;11(6):1609. doi: 10.3390/biomedicines11061609. Biomedicines. 2023. PMID: 37371704 Free PMC article.

