Exercise hormone irisin is a critical regulator of cognitive function
- PMID: 34417591
- PMCID: PMC10317538
- DOI: 10.1038/s42255-021-00438-z
Exercise hormone irisin is a critical regulator of cognitive function
Erratum in
-
Author Correction: Exercise hormone irisin is a critical regulator of cognitive function.Nat Metab. 2021 Oct;3(10):1432. doi: 10.1038/s42255-021-00476-7. Nat Metab. 2021. PMID: 34621079 No abstract available.
Abstract
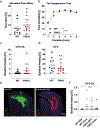
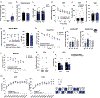
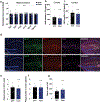
Identifying secreted mediators that drive the cognitive benefits of exercise holds great promise for the treatment of cognitive decline in ageing or Alzheimer's disease (AD). Here, we show that irisin, the cleaved and circulating form of the exercise-induced membrane protein FNDC5, is sufficient to confer the benefits of exercise on cognitive function. Genetic deletion of Fndc5/irisin (global Fndc5 knock-out (KO) mice; F5KO) impairs cognitive function in exercise, ageing and AD. Diminished pattern separation in F5KO mice can be rescued by delivering irisin directly into the dentate gyrus, suggesting that irisin is the active moiety. In F5KO mice, adult-born neurons in the dentate gyrus are morphologically, transcriptionally and functionally abnormal. Importantly, elevation of circulating irisin levels by peripheral delivery of irisin via adeno-associated viral overexpression in the liver results in enrichment of central irisin and is sufficient to improve both the cognitive deficit and neuropathology in AD mouse models. Irisin is a crucial regulator of the cognitive benefits of exercise and is a potential therapeutic agent for treating cognitive disorders including AD.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
COMPETING INTERESTS STATEMENT
The authors declare the following competing interests: B.M.S and C.D.W. hold a patent related to irisin (WO2015051007A1). B.M.S. and C.D.W. are academic co-founders and consultants for Aevum Therapeutics. C.D.W. has a financial interest in Aevum Therapeutics, a company developing drugs which harness the protective molecular mechanisms of exercise to treat neurodegenerative and neuromuscular disorders. Dr. Wrann’s interests were reviewed and are managed by Massachusetts General Hospital and Mass General Brigham in accordance with their conflict of interest policies. The other authors declare no competing interests.
Figures















References
-
- Colcombe S & Kramer AF Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci 14, 125–130 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

