GABAA receptors: structure, function, pharmacology, and related disorders
- PMID: 34417930
- PMCID: PMC8380214
- DOI: 10.1186/s43141-021-00224-0
GABAA receptors: structure, function, pharmacology, and related disorders
Abstract
Background: γ-Aminobutyric acid sub-type A receptors (GABAARs) are the most prominent inhibitory neurotransmitter receptors in the CNS. They are a family of ligand-gated ion channel with significant physiological and therapeutic implications.
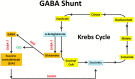
Main body: GABAARs are heteropentamers formed from a selection of 19 subunits: six α (alpha1-6), three β (beta1-3), three γ (gamma1-3), three ρ (rho1-3), and one each of the δ (delta), ε (epsilon), π (pi), and θ (theta) which result in the production of a considerable number of receptor isoforms. Each isoform exhibits distinct pharmacological and physiological properties. However, the majority of GABAARs are composed of two α subunits, two β subunits, and one γ subunit arranged as γ2β2α1β2α1 counterclockwise around the center. The mature receptor has a central chloride ion channel gated by GABA neurotransmitter and modulated by a variety of different drugs. Changes in GABA synthesis or release may have a significant effect on normal brain function. Furthermore, The molecular interactions and pharmacological effects caused by drugs are extremely complex. This is due to the structural heterogeneity of the receptors, and the existence of multiple allosteric binding sites as well as a wide range of ligands that can bind to them. Notably, dysfunction of the GABAergic system contributes to the development of several diseases. Therefore, understanding the relationship between GABAA receptor deficits and CNS disorders thus has a significant impact on the discovery of disease pathogenesis and drug development.
Conclusion: To date, few reviews have discussed GABAA receptors in detail. Accordingly, this review aims to summarize the current understanding of the structural, physiological, and pharmacological properties of GABAARs, as well as shedding light on the most common associated disorders.
Keywords: Allosteric modulation; Alzheimer’s disease; Autism spectrum disorder; Barbiturates; Benzodiazepine; Epilepsy; GABA; GABAAR; Schizophrenia.
© 2021. The Author(s).
Conflict of interest statement
Not applicable
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
