Benzodiazepine administration patterns before escalation to second-line medications in pediatric refractory convulsive status epilepticus
- PMID: 34418087
- PMCID: PMC9292193
- DOI: 10.1111/epi.17043
Benzodiazepine administration patterns before escalation to second-line medications in pediatric refractory convulsive status epilepticus
Abstract
Objective: This study was undertaken to evaluate benzodiazepine (BZD) administration patterns before transitioning to non-BZD antiseizure medication (ASM) in pediatric patients with refractory convulsive status epilepticus (rSE).
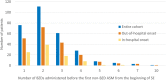
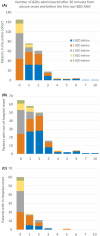
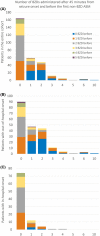
Methods: This retrospective multicenter study in the United States and Canada used prospectively collected observational data from children admitted with rSE between 2011 and 2020. Outcome variables were the number of BZDs given before the first non-BZD ASM, and the number of BZDs administered after 30 and 45 min from seizure onset and before escalating to non-BZD ASM.
Results: We included 293 patients with a median (interquartile range) age of 3.8 (1.3-9.3) years. Thirty-six percent received more than two BZDs before escalating, and the later the treatment initiation was after seizure onset, the less likely patients were to receive multiple BZD doses before transitioning (incidence rate ratio [IRR] = .998, 95% confidence interval [CI] = .997-.999 per minute, p = .01). Patients received BZDs beyond 30 and 45 min in 57.3% and 44.0% of cases, respectively. Patients with out-of-hospital seizure onset were more likely to receive more doses of BZDs beyond 30 min (IRR = 2.43, 95% CI = 1.73-3.46, p < .0001) and beyond 45 min (IRR = 3.75, 95% CI = 2.40-6.03, p < .0001) compared to patients with in-hospital seizure onset. Intermittent SE was a risk factor for more BZDs administered beyond 45 min compared to continuous SE (IRR = 1.44, 95% CI = 1.01-2.06, p = .04). Forty-seven percent of patients (n = 94) with out-of-hospital onset did not receive treatment before hospital arrival. Among patients with out-of-hospital onset who received at least two BZDs before hospital arrival (n = 54), 48.1% received additional BZDs at hospital arrival.
Significance: Failure to escalate from BZDs to non-BZD ASMs occurs mainly in out-of-hospital rSE onset. Delays in the implementation of medical guidelines may be reduced by initiating treatment before hospital arrival and facilitating a transition to non-BZD ASMs after two BZD doses during handoffs between prehospital and in-hospital settings.
Keywords: benzodiazepine; epilepsy; pediatric; seizure; status epilepticus; treatment.
© 2021 The Authors. Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
J.N.B. has served as a consultant for Novartis. T.A.G. is funded by National Institutes of Health (NIH) grants 2U01‐NS045911, U10‐NS077311, R01‐NS053998, R01‐NS062756, R01‐NS043209, R01‐LM011124, and R01‐NS065840; has received consulting fees from Supernus, Sunovion, Eisai, and UCB; serves as an expert consultant for the US Department of Justice and has received compensation for work as an expert on medicolegal cases; and receives royalties from a patent license. E.J.N. is on the professional advisory board of the Epilepsy Foundation of America and served on an advisory board for Zogenix. J.J.R.'s spouse is an editor for UpToDate. D.T. receives research funding from Children's Miracle Network Hospitals and has previously received consultation fees from Gerson Lehrman Group, Guidepoint, IQVIA, and bioStrategies Group. R.S. receives research funding from PCORI, NIH, the Pediatric Epilepsy Research Foundation, and the University of Michigan; receives royalties from UpToDate for authorship of topics related to neonatal seizures, is a consultant for the Epilepsy Study Consortium, and is an associate editor for
Figures
Similar articles
-
Pediatric Status Epilepticus: Treat Early and Avoid Delays.Paediatr Drugs. 2023 Jul;25(4):411-424. doi: 10.1007/s40272-023-00570-1. Epub 2023 May 13. Paediatr Drugs. 2023. PMID: 37178271 Review.
-
First-line medication dosing in pediatric refractory status epilepticus.Neurology. 2020 Nov 10;95(19):e2683-e2696. doi: 10.1212/WNL.0000000000010828. Epub 2020 Sep 10. Neurology. 2020. PMID: 32913024 Free PMC article.
-
Inadequate benzodiazepine dosing may result in progression to refractory and non-convulsive status epilepticus.Epileptic Disord. 2018 Aug 1;20(4):265-269. doi: 10.1684/epd.2018.0987. Epileptic Disord. 2018. PMID: 30113012
-
Factors associated with treatment delays in pediatric refractory convulsive status epilepticus.Neurology. 2018 May 8;90(19):e1692-e1701. doi: 10.1212/WNL.0000000000005488. Epub 2018 Apr 11. Neurology. 2018. PMID: 29643084 Free PMC article.
-
Pharmacotherapy for Pediatric Convulsive Status Epilepticus.CNS Drugs. 2020 Jan;34(1):47-63. doi: 10.1007/s40263-019-00690-8. CNS Drugs. 2020. PMID: 31879852 Free PMC article. Review.
Cited by
-
Pediatric Status Epilepticus: Treat Early and Avoid Delays.Paediatr Drugs. 2023 Jul;25(4):411-424. doi: 10.1007/s40272-023-00570-1. Epub 2023 May 13. Paediatr Drugs. 2023. PMID: 37178271 Review.
-
Epileptic Status in a PEDiatric cohort (ESPED) requiring intensive care treatment: A multicenter, national, two-year prospective surveillance study.Epilepsia Open. 2023 Jun;8(2):411-424. doi: 10.1002/epi4.12707. Epub 2023 Feb 20. Epilepsia Open. 2023. PMID: 36764666 Free PMC article.
-
An Italian Survey on the Management of Pediatric Convulsive Status Epilepticus: More Than Just a Pharmacological Choice.Brain Behav. 2025 Apr;15(4):e70433. doi: 10.1002/brb3.70433. Brain Behav. 2025. PMID: 40165501 Free PMC article.
-
Treatment of pediatric convulsive status epilepticus.Front Neurol. 2023 Jun 29;14:1175370. doi: 10.3389/fneur.2023.1175370. eCollection 2023. Front Neurol. 2023. PMID: 37456627 Free PMC article. Review.
-
Using an in-situ Simulation Model to Identify Deviations from Guideline-Based Management of Pediatric Status Epilepticus in Community Emergency Departments.Open Access Emerg Med. 2025 May 7;17:165-171. doi: 10.2147/OAEM.S507770. eCollection 2025. Open Access Emerg Med. 2025. PMID: 40357448 Free PMC article.
References
-
- Chin RF, Neville BG, Peckham C, Bedford H, Wade A, Scott RC, et al. Incidence, cause, and short‐term outcome of convulsive status epilepticus in childhood: prospective population‐based study. Lancet. 2006;368:222–9. - PubMed
-
- Coeytaux A, Jallon P, Galobardes B, Morabia A. Incidence of status epilepticus in French‐speaking Switzerland: (EPISTAR). Neurology. 2000;55:693–7. - PubMed
-
- DeLorenzo RJ, Hauser WA, Towne AR, Boggs JG, Pellock JM, Penberthy L, et al. A prospective, population‐based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. 1996;46:1029–35. - PubMed
-
- Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Incidence of status epilepticus in Rochester, Minnesota, 1965–1984. Neurology. 1998;50:735–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

