Baseline severity and the prediction of placebo response in clinical trials for alcohol dependence: A meta-regression analysis to develop an enrichment strategy
- PMID: 34418121
- PMCID: PMC9291112
- DOI: 10.1111/acer.14670
Baseline severity and the prediction of placebo response in clinical trials for alcohol dependence: A meta-regression analysis to develop an enrichment strategy
Abstract
Background: There is considerable unexplained variability in alcohol abstinence rates (AR) in the placebo groups of randomized controlled trials (RCTs) for alcohol dependence (AD). This is of particular interest because placebo responses correlate negatively with treatment effect size. Recent evidence suggests that the placebo response is lower in very heavy drinkers who show no "spontaneous improvement" prior to treatment initiation (high-severity population) than in a mild-severity population and in studies with longer treatment duration. We systematically investigated the relationship between population severity, treatment duration, and the placebo response in AR to inform a strategy aimed at reducing the placebo response and thereby increasing assay sensitivity in RCTs for AD.
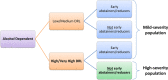
Methods: We conducted a systematic literature review on placebo-controlled RCTs for AD.We assigned retained RCTs to high- or mild-severity groups of studies based on baseline drinking risk levels and abstinence duration before treatment initiation. We tested the effects of population severity and treatment duration on the placebo response in AR using meta-regression analysis.
Results: Among the 19 retained RCTs (comprising 1996 placebo-treated patients), 11 trials were high-severity and 8 were mild-severity RCTs. The between-study variability in AR was lower in the high-severity than in the mild-severity studies (interquartile range: 7.4% vs. 20.9%). The AR in placebo groups was dependent on population severity (p = 0.004) and treatment duration (p = 0.017) and was lower in the high-severity studies (16.8% at 3 months) than the mild-severity studies (36.7% at 3 months).
Conclusions: Pharmacological RCTs for AD should select high-severity patients to decrease the magnitude and variability in the placebo effect and and improve the efficiency of drug development efforts for AD.
Keywords: alcohol dependence; alcohol use disorder; placebo response; predictor; randomized controlled trials.
© 2021 The Authors. Alcoholism: Clinical & Experimental Research published by Wiley Periodicals LLC on behalf of Research Society on Alcoholism.
Conflict of interest statement
Giovanni Addolorato served as a consultant for Ortho‐McNeil Janssen Scientific Affairs, LLC, and D&A Pharma, and was paid for his consulting services. He has received lecture fees from D&A Pharma. Henri‐Jean Aubin reports being member of advisory boards or DSMB for Bioprojet, and Ethypharm and has received sponsorship to attend scientific meetings, speaker honoraria, or consultancy fees from Bioprojet, D&A Pharma, Ethypharm, Kinnov Pharmaceuticals, and Lundbeck. He is also member of the American Society of Clinical Psychopharmacology's Alcohol Clinical Trials Initiative (ACTIVE), which was supported in the last 3 years by Alkermes, Amygdala Neurosciences, Arbor Pharmaceuticals, Indivior, Lundbeck, Mitsubishi, and Otsuka. Rolland Benjamin received fees from Ethypharm and Lundbeck. David Nutt reports personal fees from D&A Pharma and Lundbeck and is a director of Alcarelle. Wim van den Brink reported personal fees from D&A Pharma, Kinnov Therapeutics, Bioproject, Lundbeck, Novartis, Indivior, Angelini, Mundipharma, Takeda, and Opiant Inc. Maurice Dematteis has provided expert advice to Bouchara‐Recordati Laboratories, Camurus, Indivior, Lundbeck, and D&APharma and received fees for lectures from Bouchara‐Recordati Laboratories, Lundbeck, Camurus, and Indivior. Antony Gual reports grants from Novartis and D&A Pharma. Lorenzo Leggio is a U.S. federal employee and is supported by the NIDA and NIAAA intramural research programs. He has also received royalties from Routledge Press (textbook) and honoraria from the UK Medical Council on Alcoholism (Editor‐in‐Chief for Alcohol and Alcoholism). Jürgen Rehm reported personal fees from D&A Pharma and Lundbeck. Rainer Spanagel reported grants from Horizon 2020 program, Era‐NET NEURON, BMBF, Deutsche Forschungsgemeinschaft (DFG) and personal fees from EMCCDA and D&A Pharma during the conduct of the study. Bruno Scherrer reported fees from D&A pharma, DNDI, HRA Pharma, and other pharmaceutical organizations. JG is employed by D&A Pharma which was one sponsor of this study. RP and QR were employed by D&A Pharma. The other funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Figures


References
-
- Anton, R.F. , Moak, D.H. , Waid, L.R. , Latham, P.K. , Malcolm, R.J. & Dias, J.K. (1999) Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo‐controlled trial. American Journal of Psychiatry, 156, 1758–1764. - PubMed
-
- Anton, R.F. , Moak, D.H. , Latham, P. , Waid, L.R. , Myrick, H. , Voronin, K. et al. (2005) Naltrexone combined with either cognitive behavioral or motivational enhancement therapy for alcohol dependence. Journal of Clinical Psychopharmacology, 25, 349–357. - PubMed
-
- Baltieri, D.A. & de Andrade, A.G. (2003) Efficacy of acamprosate in the treatment of alcohol‐dependent outpatients. Revista Brasileira de Psiquiatria, 25, 156–159. - PubMed
-
- Baltieri, D.A. , Daró, F.R. , Ribeiro, P.L. & de Andrade, A.G. (2008) Comparing topiramate with naltrexone in the treatment of alcohol dependence*. Addiction, 103, 2035–2044. - PubMed
-
- de Bejczy, A. , Löf, E. , Walther, L. , Guterstam, J. , Hammarberg, A. , Asanovska, G. et al. (2015) Varenicline for treatment of alcohol dependence: a randomized, placebo‐controlled trial. Alcoholism, Clinical and Experimental Research, 39, 2189–2199. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

