Extracellular vesicle-based interorgan transport of mitochondria from energetically stressed adipocytes
- PMID: 34418352
- PMCID: PMC8429176
- DOI: 10.1016/j.cmet.2021.08.002
Extracellular vesicle-based interorgan transport of mitochondria from energetically stressed adipocytes
Abstract
Adipocytes undergo intense energetic stress in obesity resulting in loss of mitochondrial mass and function. We have found that adipocytes respond to mitochondrial stress by rapidly and robustly releasing small extracellular vesicles (sEVs). These sEVs contain respiration-competent, but oxidatively damaged mitochondrial particles, which enter circulation and are taken up by cardiomyocytes, where they trigger a burst of ROS. The result is compensatory antioxidant signaling in the heart that protects cardiomyocytes from acute oxidative stress, consistent with a preconditioning paradigm. As such, a single injection of sEVs from energetically stressed adipocytes limits cardiac ischemia/reperfusion injury in mice. This study provides the first description of functional mitochondrial transfer between tissues and the first vertebrate example of "inter-organ mitohormesis." Thus, these seemingly toxic adipocyte sEVs may provide a physiological avenue of potent cardio-protection against the inevitable lipotoxic or ischemic stresses elicited by obesity.
Keywords: adipocyte; cardiovascular disease; diabetes; exosomes; extracellular vesicles; mitochondria; mitochondrial dysfunction; mitohormesis; obesity; stress response.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
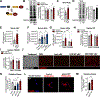


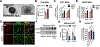



Comment in
-
Mitochondria transported from adipocytes in extracellular vesicles.Nat Rev Endocrinol. 2021 Nov;17(11):637. doi: 10.1038/s41574-021-00563-5. Nat Rev Endocrinol. 2021. PMID: 34480117 No abstract available.
-
Metabolically stressed adipocytes: mediators of cardioprotection via extracellular vesicle-mediated transport of oxidatively damaged mitochondria.ExRNA. 2022 Jan;4:3. doi: 10.21037/exrna-21-31. Epub 2022 Jan 25. ExRNA. 2022. PMID: 35211656 Free PMC article. No abstract available.
References
-
- Bank IE, Timmers L, Gijsberts CM, Zhang YN, Mosterd A, Wang JW, Chan MY, De Hoog V, Lim SK, Sze SK, et al. (2015). The diagnostic and prognostic potential of plasma extracellular vesicles for cardiovascular disease. Expert Rev Mol Diagn 15, 1577–1588. - PubMed
Publication types
MeSH terms
Grants and funding
- S10 OD021685/OD/NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- S10 OD021684/OD/NIH HHS/United States
- K99 DK122019/DK/NIDDK NIH HHS/United States
- P01 AG051459/AG/NIA NIH HHS/United States
- R01 DK099110/DK/NIDDK NIH HHS/United States
- R01 DK055758/DK/NIDDK NIH HHS/United States
- R01 DK127274/DK/NIDDK NIH HHS/United States
- RC2 DK118620/DK/NIDDK NIH HHS/United States
- K01 DK125447/DK/NIDDK NIH HHS/United States
- R00 DK122019/DK/NIDDK NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

