Safety and efficacy of tolebrutinib, an oral brain-penetrant BTK inhibitor, in relapsing multiple sclerosis: a phase 2b, randomised, double-blind, placebo-controlled trial
- PMID: 34418400
- PMCID: PMC8434816
- DOI: 10.1016/S1474-4422(21)00237-4
Safety and efficacy of tolebrutinib, an oral brain-penetrant BTK inhibitor, in relapsing multiple sclerosis: a phase 2b, randomised, double-blind, placebo-controlled trial
Abstract
Background: Tolebrutinib is an oral, CNS-penetrant, irreversible inhibitor of Bruton's tyrosine kinase, an enzyme expressed in B lymphocytes and myeloid cells including microglia, which are major drivers of inflammation in multiple sclerosis. We aimed to determine the dose-response relationship between tolebrutinib and the reduction in new active brain MRI lesions in patients with relapsing multiple sclerosis.
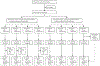
Methods: We did a 16-week, phase 2b, randomised, double-blind, placebo-controlled, crossover, dose-finding trial at 40 centres (academic sites, specialty clinics, and general neurology centres) in ten countries in Europe and North America. Eligible participants were adults aged 18-55 years with diagnosed relapsing multiple sclerosis (either relapsing-remitting or relapsing secondary progressive multiple sclerosis), and one or more of the following criteria: at least one relapse within the previous year, at least two relapses within the previous 2 years, or at least one active gadolinium-enhancing brain lesion in the 6 months before screening. Exclusion criteria included a diagnosis of primary progressive multiple sclerosis or a diagnosis of secondary progressive multiple sclerosis without relapse. We used a two-step randomisation process to randomly assign eligible participants (1:1) to two cohorts, then further randomly assign participants in each cohort (1:1:1:1) to four tolebrutinib dose groups (5, 15, 30, and 60 mg administered once daily as an oral tablet). Cohort 1 received tolebrutinib for 12 weeks, then matched placebo (ie, identical looking tablets) for 4 weeks; cohort 2 received 4 weeks of placebo followed by 12 weeks of tolebrutinib. Participants and investigators were masked for dose and tolebrutinib-placebo administration sequence; investigators, study team members, and study participants did not have access to unmasked data. MRI scans were done at screening and every 4 weeks over 16 weeks. The primary efficacy endpoint was the number of new gadolinium-enhancing lesions detected on the scan done after 12 weeks of tolebrutinib treatment (assessed at week 12 for cohort 1 and week 16 for cohort 2), relative to the scan done 4 weeks previously, and compared with the lesions accumulated during 4 weeks of placebo run-in period in cohort 2. Efficacy data were analysed in a modified intention-to-treat population, using a two-step multiple comparison procedure with modelling analysis. Safety was assessed for all participants who received at least one dose of study drug. This trial is registered with ClinicalTrials.gov (NCT03889639), EudraCT (2018-003927-12), and WHO (U1111-1220-0572), and has been completed.
Findings: Between May 14, 2019, and Jan 2, 2020, we enrolled and randomly assigned 130 participants to tolebrutinib: 33 to 5 mg, 32 to 15 mg, 33 to 30 mg, and 32 to 60 mg. 129 (99%) completed the treatment regimen and 126 were included in the primary analysis. At treatment week 12, there was a dose-dependent reduction in the number of new gadolinium-enhancing lesions (mean [SD] lesions per patient: placebo, 1·03 [2·50]; 5 mg, 1·39 [3·20]; 15 mg, 0·77 [1·48]; 30 mg, 0·76 [3·31]; 60 mg, 0·13 [0·43]; p=0·03). One serious adverse event was reported (one patient in the 60 mg group was admitted to hospital because of a multiple sclerosis relapse). The most common non-serious adverse event during tolebrutinib treatment was headache (in one [3%] of 33 in the 5 mg group; three [9%] of 32 in the 15 mg group; one [3%] of 33 in the 30 mg group; and four [13%] of 32 in the 60 mg group). No safety-related discontinuations or treatment-related deaths occurred.
Interpretation: 12 weeks of tolebrutinib treatment led to a dose-dependent reduction in new gadolinium-enhancing lesions, the 60 mg dose being the most efficacious, and the drug was well tolerated. Reduction of acute inflammation, combined with the potential to modulate the immune response within the CNS, provides a scientific rationale to pursue phase 3 clinical trials of tolebrutinib in patients with relapsing and progressive forms of multiple sclerosis.
Funding: Sanofi.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests DSR is supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke and has received research support from Vertex. DLA reports consulting fees from Acorda Therapeutics, Biogen, Celgene, Genentech, GeNeuro, F Hoffmann-La Roche, Merck, Novartis, Roche, Sanofi, Teva, and Wave Life Science; financial support for research activities from Biogen Idec Canada, Immunotec, Novartis Canada, and Novartis Global Medical Affairs; and personal compensation from NeuroRx Research. PV reports consulting or speaking fees and research support from Biogen, Celgene, Merck, Novartis, Roche, Sanofi, and Teva. AB-O reports consultancy fees or grant support from Biogen Idec, Genentech, GlaxoSmithKline, Merck/EMD Serono, Novartis, Receptos, Roche, and Sanofi. RJF reports consulting fees from AB Science, Actelion, Biogen, Celgene, EMD Serono, Genentech, Immunic Therapeutics, Novartis, Sanofi, Teva, and TG Therapeutics; research support from Biogen and Novartis; and participating on advisory boards for Actelion, Biogen, Immunic Therapeutics, and Novartis. AM, TT, EW, and XZ report being employees of Sanofi. AT reports compensation for consulting, serving on a scientific advisory board, speaking, or other activities from Biogen Idec, Roche, Sanofi, and Teva Innovation; and grant or research support from Roche and Sanofi. MM and FAK declare no competing interests.
Figures


Comment in
-
BTK inhibitors as potential therapies for multiple sclerosis.Lancet Neurol. 2021 Sep;20(9):689-691. doi: 10.1016/S1474-4422(21)00250-7. Lancet Neurol. 2021. PMID: 34418385 No abstract available.
-
Safety and efficacy of tolebrutinib, an oral brain-penetrant BTK inhibitor, in relapsing multiple sclerosis: A phase 2b, randomized, double-blind, placebo-controlled trial by Daniel S Reich et Al.Mult Scler Relat Disord. 2023 Sep;77:104850. doi: 10.1016/j.msard.2023.104850. Epub 2023 Jun 25. Mult Scler Relat Disord. 2023. PMID: 37423047 No abstract available.
References
-
- Hauser SL, Bar-Or A, Cohen JA, et al.Ofatumumab versus Teriflunomide in Multiple Sclerosis. The New England journal of medicine 2020; 383(6): 546–57. - PubMed
-
- Hauser SL, Bar-Or A, Comi G, et al.Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. The New England journal of medicine 2017; 376(3): 221–34. - PubMed
-
- Montalban X, Arnold DL, Weber MS, et al.Placebo-controlled trial of an oral BTK inhibitor in multiple sclerosis. The New England journal of medicine 2019; 380(25): 2406–17. - PubMed
-
- Bunai T, Terada T, Kono S, et al.Neuroinflammation following disease modifying therapy in multiple sclerosis: A pilot positron emission tomography study. J Neurol Sci 2018; 385: 30–3. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

