Pancreatic Tumor Microenvironment Factor Promotes Cancer Stemness via SPP1-CD44 Axis
- PMID: 34418441
- PMCID: PMC10069715
- DOI: 10.1053/j.gastro.2021.08.023
Pancreatic Tumor Microenvironment Factor Promotes Cancer Stemness via SPP1-CD44 Axis
Abstract
Background & aims: Tumor-microenvironment factors and cancer stem cells (CSCs) play a critical role in the aggressiveness of pancreatic cancer (PC). However, the degree to which tumor-microenvironment factors promote stemness remains unexplored. Here, we examined whether cancer-associated fibroblasts (CAFs) promote CSC features in PC.
Methods: PC cells were treated long-term (30, 60, and 90 days) with conditioned media (CM)-derived from normal human fibroblasts (NFs) and CAFs. The stemness features of tumorsphere formation and stemness populations, along with CSCs markers, were analyzed using 2-dimensional and 3-dimensional sodium alginate bead-based co-culture models. Immunohistochemistry and immunofluorescence staining were performed for CSCs and fibroblast markers in autochthonous KrasG12D/+; Trp53R172H/+; Pdx1-Cre mice and human pancreatic tumors. Polymerase chain reaction array and gene knockdown were performed to identify the mechanism of stemness enrichment.
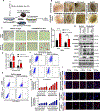
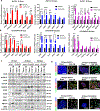
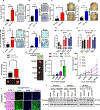
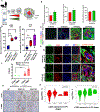
Results: Long-term treatment of PC cells with CAF-CM enriched stemness, as indicated by significantly higher CD44+, ALDH+, and AF+ populations in PC cells. Increased tumorsphere formation and elevated CSC, self-renewal, and drug-resistance markers in CAF-CM-treated PC cells were observed. In addition, CAFs co-cultured with PC cells in the 3-dimensional model showed a substantial increase in stemness features. CD44 and α-smooth muscle actin were positively correlated and their expressions progressively increased from the early to late stages of KrasG12D/+; Trp53R172H/+; Pdx1-Cre mouse and human pancreatic tumors. Osteopontin/secreted phosphoprotein 1 was identified as the top differentially overexpressed gene in CAF-CM-treated PC cells and knockdown of osteopontin/secreted phosphoprotein 1 significantly reduced stemness characteristics in CAF-CM-treated PC cells.
Conclusions: Our data uncovered novel insight into the interplay between CAF and enrichment of stemness population through the osteopontin/secreted phosphoprotein 1-CD44 axis in PC.
Keywords: CD44; Cancer Stem Cells; Cancer-Associated Fibroblast; OPN/SPP1; Pancreatic Cancer.
Published by Elsevier Inc.
Conflict of interest statement
Conflicts of interest
SKB is one of the co-founders of Sanguine Diagnostics and Therapeutics, Inc. The other authors disclosed no potential conflicts of interest.
Figures



Comment in
-
Interplay of Tumor Microenvironment Factors and Cancer Stem Cell Enrichment in Pancreatic Ductal Adenocarcinoma.Gastroenterology. 2021 Dec;161(6):1800-1802. doi: 10.1053/j.gastro.2021.09.024. Epub 2021 Sep 15. Gastroenterology. 2021. PMID: 34536452 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

