Polymeric and lipid nanoparticles for delivery of self-amplifying RNA vaccines
- PMID: 34418521
- PMCID: PMC8412240
- DOI: 10.1016/j.jconrel.2021.08.029
Polymeric and lipid nanoparticles for delivery of self-amplifying RNA vaccines
Abstract
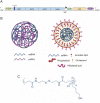
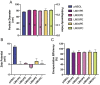
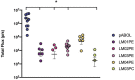
Self-amplifying RNA (saRNA) is a next-generation vaccine platform, but like all nucleic acids, requires a delivery vehicle to promote cellular uptake and protect the saRNA from degradation. To date, delivery platforms for saRNA have included lipid nanoparticles (LNP), polyplexes and cationic nanoemulsions; of these LNP are the most clinically advanced with the recent FDA approval of COVID-19 based-modified mRNA vaccines. While the effect of RNA on vaccine immunogenicity is well studied, the role of biomaterials in saRNA vaccine effectiveness is under investigated. Here, we tested saRNA formulated with either pABOL, a bioreducible polymer, or LNP, and characterized the protein expression and vaccine immunogenicity of both platforms. We observed that pABOL-formulated saRNA resulted in a higher magnitude of protein expression, but that the LNP formulations were overall more immunogenic. Furthermore, we observed that both the helper phospholipid and route of administration (intramuscular versus intranasal) of LNP impacted the vaccine immunogenicity of two model antigens (influenza hemagglutinin and SARS-CoV-2 spike protein). We observed that LNP administered intramuscularly, but not pABOL or LNP administered intranasally, resulted in increased acute interleukin-6 expression after vaccination. Overall, these results indicate that delivery systems and routes of administration may fulfill different delivery niches within the field of saRNA genetic medicines.
Keywords: Immunogenicity; Lipid nanoparticle; Polyplex; Protein expression; Replicon; SARS-CoV-2; Self-amplifying RNA; Vaccine.
Copyright © 2021 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare the following competing financial interest(s): AKB, YZ, RJS, and MMS are co-inventors on a patent resulting from this work. AT, NJ and AG are employees of Precision NanoSystems, Inc. The remaining authors declare no competing interests.
Figures





References
-
- Perri S., Greer C.E., Thudium K., Doe B., Legg H., Liu H., Romero R.E., Tang Z., Bin Q., Dubensky T.W., Vajdy M., Otten G.R., Polo J.M. An Alphavirus replicon particle chimera derived from Venezuelan equine encephalitis and Sindbis viruses is a potent gene-based vaccine delivery vector. J. Virol. 2003;77:10394. - PMC - PubMed
-
- Vogel A.B., Lambert L., Kinnear E., Busse D., Erbar S., Reuter K.C., Wicke L., Perkovic M., Beissert T., Haas H., Reece S.T., Sahin U., Tregoning J.S. Self-amplifying RNA vaccines give equivalent protection against influenza to mRNA vaccines but at much lower doses. Mol. Ther. 2018;26:446–455. - PMC - PubMed
-
- Blakney A.K., McKay P.F., Christensen D., Yus B.I., Aldon Y., Follmann F., Shattock R.J. Effects of cationic adjuvant formulation particle type, fluidity and immunomodulators on delivery and immunogenicity of saRNA. J. Control. Release. 2019;304:65–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

