Recent insights into the role of glia and oxidative stress in Alzheimer's disease gained from Drosophila
- PMID: 34418791
- PMCID: PMC8854453
- DOI: 10.1016/j.conb.2021.07.012
Recent insights into the role of glia and oxidative stress in Alzheimer's disease gained from Drosophila
Abstract
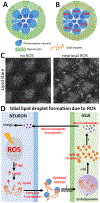
Here, we discuss findings made using Drosophila on Alzheimer's disease (AD) risk and progression. Recent studies have investigated the mechanisms underlying glia-mediated neuroprotection in AD. First, we discuss a novel mechanism of glial lipid droplet formation that occurs in response to elevated reactive oxygen species in neurons. The data suggest that disruptions to this process contribute to AD risk. We further discuss novel mechanistic insights into glia-mediated Aβ42-clearance made using the fly. Finally, we highlight work that provides evidence that the aberrant accumulation of reactive oxygen species in AD may not just be a consequence of disease but contribute to disease progression as well. Cumulatively, the discussed studies highlight recent, relevant discoveries in AD made using Drosophila.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement Nothing declared.
Figures

References
-
- Soria Lopez JA, González HM, Léger GC: Chapter 13 - Alzheimer’s disease. In Handbook of Clinical Neurology. Edited by Dekosky ST, Asthana S. Elsevier; 2019:231–255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

