Tenofovir alafenamide nephrotoxicity: a case report and literature review
- PMID: 34419091
- PMCID: PMC8379835
- DOI: 10.1186/s12981-021-00380-w
Tenofovir alafenamide nephrotoxicity: a case report and literature review
Abstract
Background: Tenofovir alafenamide (TAF), a novel prodrug of tenofovir (TFV), has become the preferred drug for the treatment of HIV-1 and chronic hepatitis B infection in clinical practice. Results from clinical trials showed that it had better renal and bone mineral outcomes compared to tenofovir disoproxil fumarate (TDF). However, as we have seen with TDF, side effects from the new medication can be more prevalent and recognized after extensive use in real world situations. Sporadic cases of acute kidney injury in patients using TAF have started to emerge.
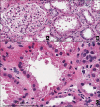
Case presentation: We report a case of 49-year-old Thai, HIV treatment-experienced female with hypertension presented with worsening renal function after switching her antiretroviral regimen from TDF, emtricitabine (FTC), and lopinavir/ritonavir (LPV/r) to TAF, FTC and dolutegravir (DTG) for 3 months. Kidney biopsy showed distinctive picture of tenofovir nephrotoxicity with acute tubular injury and mitochondrial injury. The possible causes of acute kidney injury and nephrotoxicity from TAF for this patient were discussed. We have extensively reviewed all published case reports of TAF-associated nephrotoxicity and summarized the essential information in this article.
Conclusion: Although TAF has less nephrotoxicity compared with TDF; renal function should always be monitored after the initiation of both drugs. Future large cohort studies are required to identify the risk factors of TAF-associated nephrotoxicity and to design an effective preventive strategy.
Keywords: Acute kidney injury; Antiretroviral therapy; Case report; HIV; Mitochondria; Nephrotoxicity; Renal pathology; Tenofovir alafenamide.
© 2021. The Author(s).
Conflict of interest statement
AA has received honorarium for consultation from ViiV Healthcare. The rest of the authors declare no relevant financial interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

