Evaluation of the effectiveness of an incentive strategy on the questionnaire response rate in parents of premature babies: a randomised controlled Study Within A Trial (SWAT) nested within SIFT
- PMID: 34419121
- PMCID: PMC8379785
- DOI: 10.1186/s13063-021-05515-y
Evaluation of the effectiveness of an incentive strategy on the questionnaire response rate in parents of premature babies: a randomised controlled Study Within A Trial (SWAT) nested within SIFT
Abstract
Background: Loss to follow-up resulting in missing outcomes compromises the validity of trial results by reducing statistical power, negatively affecting generalisability and undermining assumptions made at analysis, leading to potentially biased and misleading results. Evidence that incentives are effective at improving response rates exists, but there is little evidence regarding the best approach, especially in the field of perinatal medicine. The NIHR-funded SIFT trial follow-up of infants at 2 years of age provided an ideal opportunity to address this remaining uncertainty.
Methods: Participants: parents of infants from participating neonatal units in the UK and Ireland followed up for SIFT (multicentre RCT investigating two speeds of feeding in babies with gestational age at birth < 32 weeks and/or birthweight < 1500 g).
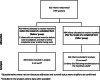
Interventions: parents were randomly allocated to receive incentives (£15 gift voucher) before or after questionnaire return. The objective was to establish whether offering an unconditional incentive in advance or promising an incentive on completion of a questionnaire (conditional) improved the response rate in parents of premature babies. The primary outcome was questionnaire response rate. Permuted block randomisation was performed (variable size blocks), stratified by SIFT allocation (slower/faster feeds) and single/multiple birth. Multiple births were given the same incentives allocation. Parents were unaware that they were in an incentives SWAT; SIFT office staff were not blinded to allocation.
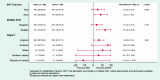
Results: Parents of 923 infants were randomised: 459 infants allocated to receive incentive before, 464 infants allocated to receive incentive after; analysis was by intention to treat. Allocation to the incentive before completion led to a significantly higher response rate, 83.0% (381/459) compared to the after-completion group, 76.1% (353/464); adjusted absolute difference of 6.8% (95% confidence interval 1.6% to 12.0%). Giving an incentive in advance is the more costly approach, but the mean difference of ~£3 per infant is small given the higher return.
Conclusions: An unconditional incentive in advance led to a significantly higher response rate compared to the promise of an incentive on completion. Against a backdrop of falling response rates to questionnaires, incentives can be an effective way to increase returns.
Trial registration: SIFT ( ISRCTN76463425 ). Registered on March 5, 2013.; SWAT registration (SWAT 69 available from http://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/FileStore/Filetoupload,864297,en.pdf ). Registered on June 27, 2016.
Keywords: Effective; Incentive; Questionnaire; Response; Unconditional.
© 2021. The Author(s).
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form at
Other authors’ competing interests: Oliver Hewer reports personal fees from London School of Hygiene and Tropical Medicine, outside the submitted work.
Figures
References
-
- Akl EA, Briel M, You JJ, Sun X, Johnston BC, Busse JW, Mulla S, Lamontagne F, Bassler D, Vera C, Alshurafa M, Katsios CM, Zhou Q, Cukierman-Yaffe T, Gangji A, Mills EJ, Walter SD, Cook DJ, Schunemann HJ, Altman DG, Guyatt GH. Potential impact on estimated treatment effects of information lost to follow-up in randomised controlled trials (LOST-IT): systematic review. BMJ. 2012;344(may18 1):e2809. doi: 10.1136/bmj.e2809. - DOI - PubMed
-
- Dillman DA. Mail and internet surveys: The tailored design method. 2. Hoboken, NJ, US: John Wiley & Sons Inc; 2007.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials