Whole-genome methylation analysis of testicular germ cells from cryptozoospermic men points to recurrent and functionally relevant DNA methylation changes
- PMID: 34419158
- PMCID: PMC8379757
- DOI: 10.1186/s13148-021-01144-z
Whole-genome methylation analysis of testicular germ cells from cryptozoospermic men points to recurrent and functionally relevant DNA methylation changes
Abstract
Background: Several studies have reported an association between male infertility and aberrant sperm DNA methylation patterns, in particular in imprinted genes. In a recent investigation based on whole methylome and deep bisulfite sequencing, we have not found any evidence for such an association, but have demonstrated that somatic DNA contamination and genetic variation confound methylation studies in sperm of severely oligozoospermic men. To find out whether testicular germ cells (TGCs) of such patients might carry aberrant DNA methylation, we compared the TGC methylomes of four men with cryptozoospermia (CZ) and four men with obstructive azoospermia, who had normal spermatogenesis and served as controls (CTR).
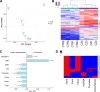
Results: There was no difference in DNA methylation at the whole genome level or at imprinted regions between CZ and CTR samples. However, using stringent filters to identify group-specific methylation differences, we detected 271 differentially methylated regions (DMRs), 238 of which were hypermethylated in CZ (binominal test, p < 2.2 × 10-16). The DMRs were enriched for distal regulatory elements (p = 1.0 × 10-6) and associated with 132 genes, 61 of which are differentially expressed at various stages of spermatogenesis. Almost all of the 67 DMRs associated with the 61 genes (94%) are hypermethylated in CZ (63/67, p = 1.107 × 10-14). As judged by single-cell RNA sequencing, 13 DMR-associated genes, which are mainly expressed during meiosis and spermiogenesis, show a significantly different pattern of expression in CZ patients. In four of these genes, the promoter is hypermethylated in CZ men, which correlates with a lower expression level in these patients. In the other nine genes, eight of which downregulated in CZ, germ cell-specific enhancers may be affected.
Conclusions: We found that impaired spermatogenesis is associated with DNA methylation changes in testicular germ cells at functionally relevant regions of the genome. We hypothesize that the described DNA methylation changes may reflect or contribute to premature abortion of spermatogenesis and therefore not appear in the mature, motile sperm.
Keywords: Cryptozoospermia; DNA methylation; Differentially methylated regions; Male infertility; Single cell RNA sequencing; Spermatogenesis; Testicular germ cells.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

