Pharmacokinetics of First-Line Drugs in Children With Tuberculosis, Using World Health Organization-Recommended Weight Band Doses and Formulations
- PMID: 34420049
- PMCID: PMC9155615
- DOI: 10.1093/cid/ciab725
Pharmacokinetics of First-Line Drugs in Children With Tuberculosis, Using World Health Organization-Recommended Weight Band Doses and Formulations
Abstract
Background: Dispersible pediatric fixed-dose combination (FDC) tablets delivering higher doses of first-line antituberculosis drugs in World Health Organization-recommended weight bands were introduced in 2015. We report the first pharmacokinetic data for these FDC tablets in Zambian and South African children in the treatment-shortening SHINE trial.
Methods: Children weighing 4.0-7.9, 8.0-11.9, 12.0-15.9, or 16.0-24.9 kg received 1, 2, 3, or 4 tablets daily, respectively (rifampicin/isoniazid/pyrazinamide [75/50/150 mg], with or without 100 mg ethambutol, or rifampicin/isoniazid [75/50 mg]). Children 25.0-36.9 kg received doses recommended for adults <37 kg (300, 150, 800, and 550 mg/d, respectively, for rifampicin, isoniazid, pyrazinamide, and ethambutol). Pharmacokinetics were evaluated after at least 2 weeks of treatment.
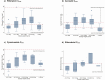
Results: In the 77 children evaluated, the median age (interquartile range) was 3.7 (1.4-6.6) years; 40 (52%) were male and 20 (26%) were human immunodeficiency virus positive. The median area under the concentration-time curve from 0 to 24 hours for rifampicin, isoniazid, pyrazinamide, and ethambutol was 32.5 (interquartile range, 20.1-45.1), 16.7 (9.2-25.9), 317 (263-399), and 9.5 (7.5-11.5) mg⋅h/L, respectively, and lower in children than in adults for rifampicin in the 4.0-7.9-, 8-11.9-, and ≥25-kg weight bands, isoniazid in the 4.0-7.9-kg and ≥25-kg weight bands, and ethambutol in all 5 weight bands. Pyrazinamide exposures were similar to those in adults.
Conclusions: Recommended weight band-based FDC doses result in lower drug exposures in children in lower weight bands and in those ≥25 kg (receiving adult doses). Further adjustments to current doses are needed to match current target exposures in adults. The use of ethambutol at the current World Health Organization-recommended doses requires further evaluation.
Keywords: antituberculosis drugs; children; dosing; pharmacokinetics; tuberculosis.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

References
-
- World Health Organization. Roadmap towards ending childhood and adolescent tuberculosis. 2nd ed. Geneva, Switzerland: World Health Organization, 2018.
-
- Thee S, Detjen A, Wahn U, Magdorf K. Rifampicin serum levels in childhood tuberculosis. Int J Tuberc Lung Dis 2009; 13:1106–11. - PubMed
-
- McIlleron H, Willemse M, Werely CJ, et al. . Isoniazid plasma concentrations in a cohort of South African children with tuberculosis: implications for international pediatric dosing guidelines. Clin Infect Dis 2009; 48:1547–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

